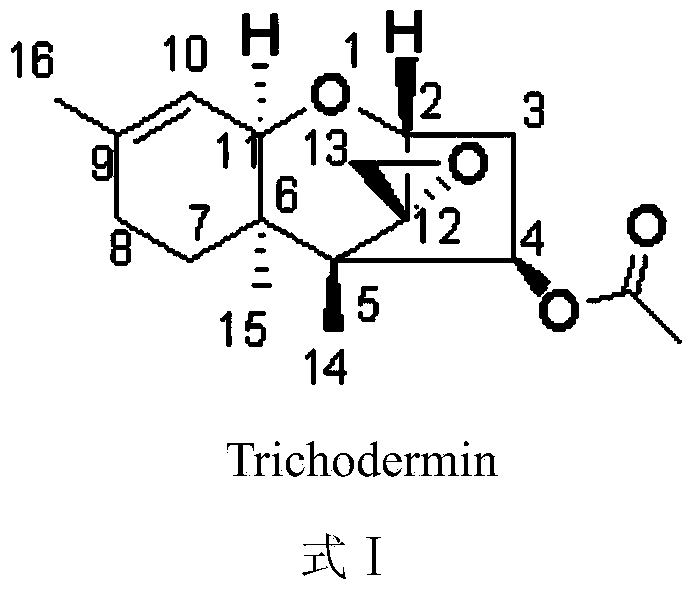
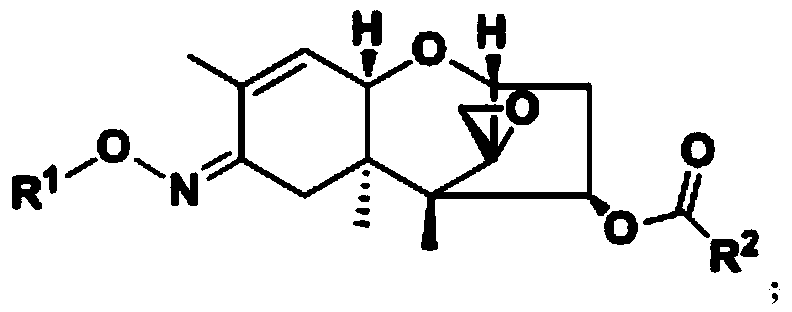
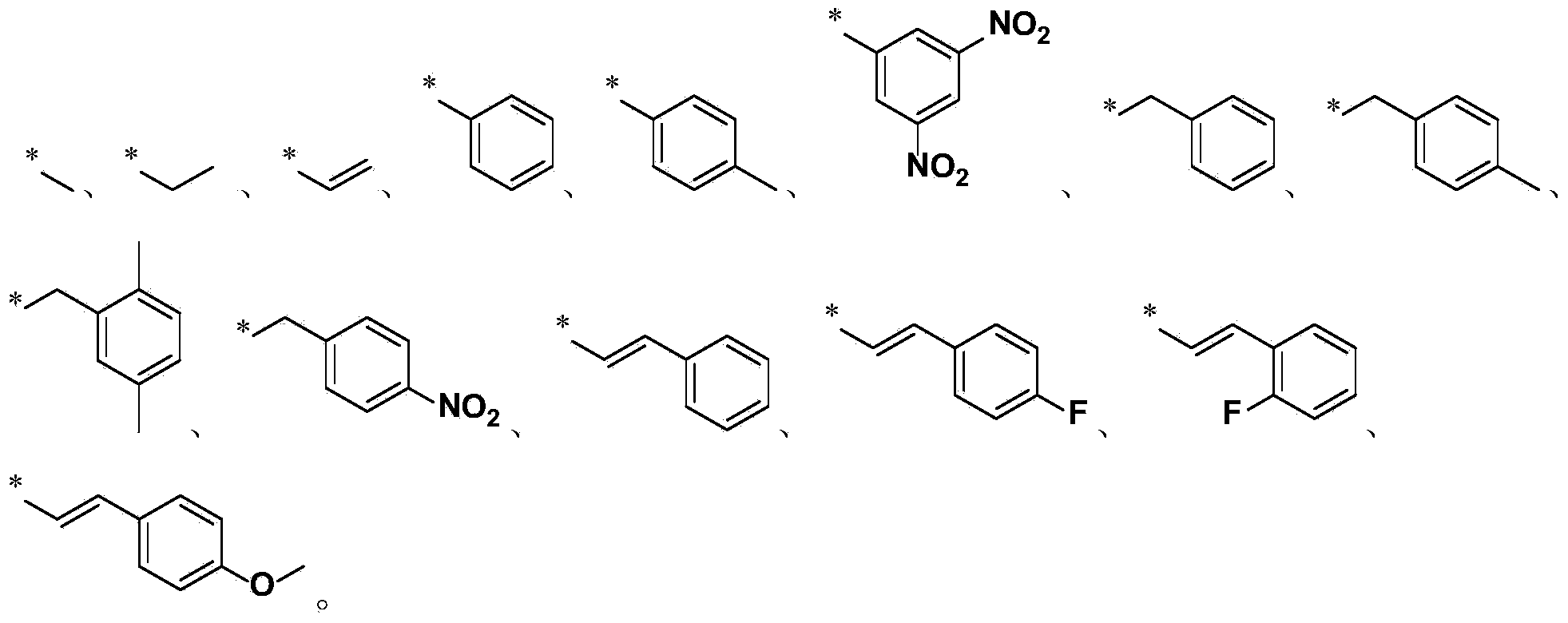
Trichodermin C8 oxime ether derivatives and application thereof
A technology of trichodermain and carbonyl trichodermain, applied in biocides, chemicals for biological control, applications, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] The synthetic method of embodiment 1, compound III, its reaction formula is:
[0086]
[0087] Concrete reaction process is as follows:
[0088] Add 0.1g of 8-carbonyltrichomycin (II), 3 equivalent molar amounts of hydroxylamine hydrochloride and 5mL of anhydrous methanol to the reaction flask, and add 3 equivalent molar amounts of potassium carbonate aqueous solution ( The mass concentration is 15-25%), and the reaction process is detected by TLC. After 5 hours, the reaction was completed. The reaction liquid was concentrated to dryness in vacuo and washed 3 times with 5% (volume concentration) dilute hydrochloric acid, 5 mL each time, and then extracted 3 times with dichloromethane (5 mL each time), and the obtained organic phase was used Anhydrous Na 2 SO 4 Drying, concentration and precipitation of white crystals, yield 88.5%.
[0089] Structural data of compound III: 1 H-NMR (500MHz, CDCl 3 ):δ8.57(s,1H,H-OH),5.88-5.84(dd,J 1 =J 2 =1.0Hz,1H,H-10),4.31-4....
Embodiment 2
[0091] Embodiment 2, the synthetic general method of compound IV, its reaction formula is as follows:
[0092]
[0093] Concrete reaction process is as follows:
[0094] Add 0.5g of trans-8-hydroxyiminotrichodermain (compound of formula III), 1.5 equivalent molar amounts of sodium hydride and 10mL of N,N-dimethylformamide into the reaction bottle, ℃) After reacting for 15 minutes, add 1.5 equivalent molar amounts of iodoalkanes (methyl iodide, ethyl iodide, propane iodide, pentyl iodide, etc.) dropwise, stir the reaction at room temperature, and detect the reaction progress by TLC. After the reaction (about 4 hours), the reaction solution was extracted three times with ethyl acetate (the amount of ethyl acetate each time was 8ml), and the obtained organic phase (located in the upper layer) was washed with water three times (the amount of water each time was 6ml), Anhydrous Na 2 SO 4 Dry and concentrate. Then, it was separated by silica gel column chromatography (using a...
Embodiment 3
[0102] Embodiment 3, a kind of synthesis method of Trichoderma C8 oxime ether derivative (A-2), its reaction formula is:
[0103]
[0104] Concrete reaction process is as follows:
[0105] Add the compound IV-1 (0.3mol) obtained by the method of Example 2, 2 equivalent molar amounts of propionic acid, 20mL CH 2 Cl 2 , adding 1 equivalent molar amount of 4-dimethylaminopyridine (DMAP) and 3 equivalent molar amounts of dicyclohexylcarbodiimide (DCC), stirring the reaction at room temperature, and detecting the reaction progress by TLC. After the reaction (about 12 hours), the reaction solution was washed three times with dilute hydrochloric acid (the volume concentration of dilute hydrochloric acid is 5%, and the dosage of each washing is 5ml), washed three times with water (the dosage of each washing is 5ml), Anhydrous Na 2 SO 4 Dry and concentrate. Then it was separated by silica gel column chromatography (eluent: ethyl acetate:petroleum ether=1:10, volume ratio) to ob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com