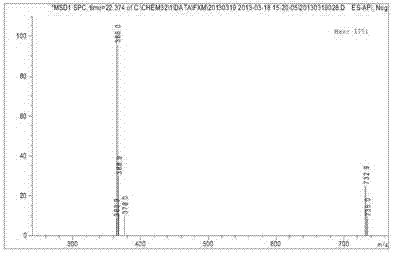
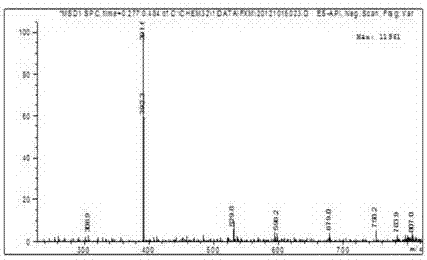
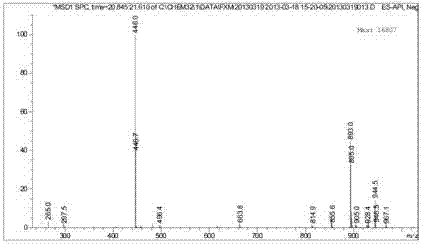
Red azo reactive dye containing sulfanilamide structure
A reactive dye and azo-based technology, applied in the direction of reactive dyes, azo dyes, organic dyes, etc., can solve the problems of dye utilization rate to be improved, good water solubility, low fiber affinity, etc., to improve dye utilization rate, excellent Color fixation rate, effect of increasing affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] 9.7g of cyanuric chloride was beaten for about 30 minutes under ice bath conditions, and 16.6g of H acid was dissolved and added dropwise to cyanuric chloride, reacted in ice bath for about 1 hour, and the pH was controlled to 3-5, and the reaction was completed (Ehrlich reagent Discoloration after detection), suction filtration, take the filtrate, weigh 8.6g sulfonamide and dissolve it in 100ml0.15mol / L hydrochloric acid solution, weigh 3.7g sodium nitrite and dissolve it in 50ml water, drop the sodium nitrite solution into the acid solution of sulfonamide , carry out diazotization reaction, add the diazonium salt obtained above to the solution obtained by H acid condensation, react in ice bath for 1 h, pH7-9, salt out after the reaction is completed, filter out the red dye, and dry it in vacuum. Yield 95%.
Embodiment 2-4
[0073] The method is the same as in Example 1, but sulfapyridine, sulfathiazole, and sulfaguanidine are used instead of sulfaguanidine to synthesize the corresponding diazonium salts, and then the corresponding red reactive dyes are obtained through the coupling reaction. Other conditions are consistent with Example 1.
[0074] Among them, the dye synthesized with sulfathiazole instead of sulfonamide (dye 1, i.e. Example 3) has the following structure:
[0075]
Embodiment 5
[0077] Weigh 9.7g of cyanuric chloride and beat for about 30 minutes under ice bath conditions; dissolve 16.6g of H acid and add it dropwise to cyanuric chloride, react in ice bath for about 1 hour, control pH3-5, pump out after the reaction is completed Filtrate and take the filtrate; dissolve 8.6g of sulfonamide under acidic conditions, slowly add the above-mentioned condensed product to the sulfonamide solution, control the pH to 6-7, react at 35°C for 4 hours, and then cool in an ice bath to obtain the condensed solution; weigh 8.6g of sulfonamide Dissolve in 100ml of 0.15mol / L hydrochloric acid solution, weigh 3.7g of sodium nitrite and dissolve in 50ml of water, drop the sodium nitrite solution into the acid solution of sulfonamide for diazotization reaction; The solution was reacted in an ice bath for 1 h, and the pH was controlled to be 7-9. After the reaction was completed, the salt was precipitated, and the red dye was obtained by suction filtration, and dried in vacu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com