Application of thiazolidone derivative in preparing anti-rhabdomyosarcoma medicines
A technology for rhabdomyosarcoma and thiazolidinone is applied in the application field of thiazolidinone derivatives in the preparation of anti-rhabdomyosarcoma drugs, and can solve problems such as adverse reactions of chemotherapy drugs, neurotoxicity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Preparation of 2-(4-hydroxyphenylimino)-5-(3-methoxybenzylidene)-4-thiazolidinone
[0035] by HCl:H 2 The ratio of O (1:4) is made into hydrochloric acid solution. Weigh 11.4g (150mmol) of ammonium thiocyanate and dissolve it in 50mL of hydrochloric acid solution, add 10.9mL (100mmol) of 4-hydroxyaniline, and heat to 85°C with stirring, the mixture becomes clear. After 12 hours of reaction, TLC monitors the reaction. After the reaction, the mixture was cooled to room temperature, and a viscous oily liquid appeared, which was extracted with ethyl acetate, and the extract was washed with 10% hydrochloric acid solution, saturated sodium chloride solution, and water successively, and the solvent was evaporated off the extract under reduced pressure to obtain Viscous oily liquid, 4-hydroxyphenylthiourea.
[0036] Add 4.24g of 4-hydroxyphenylthiourea and 9.15g of anhydrous sodium acetate into 35mL of ethanol, add 5.13g of ethyl chloroacetate under stirring condit...
Embodiment 2
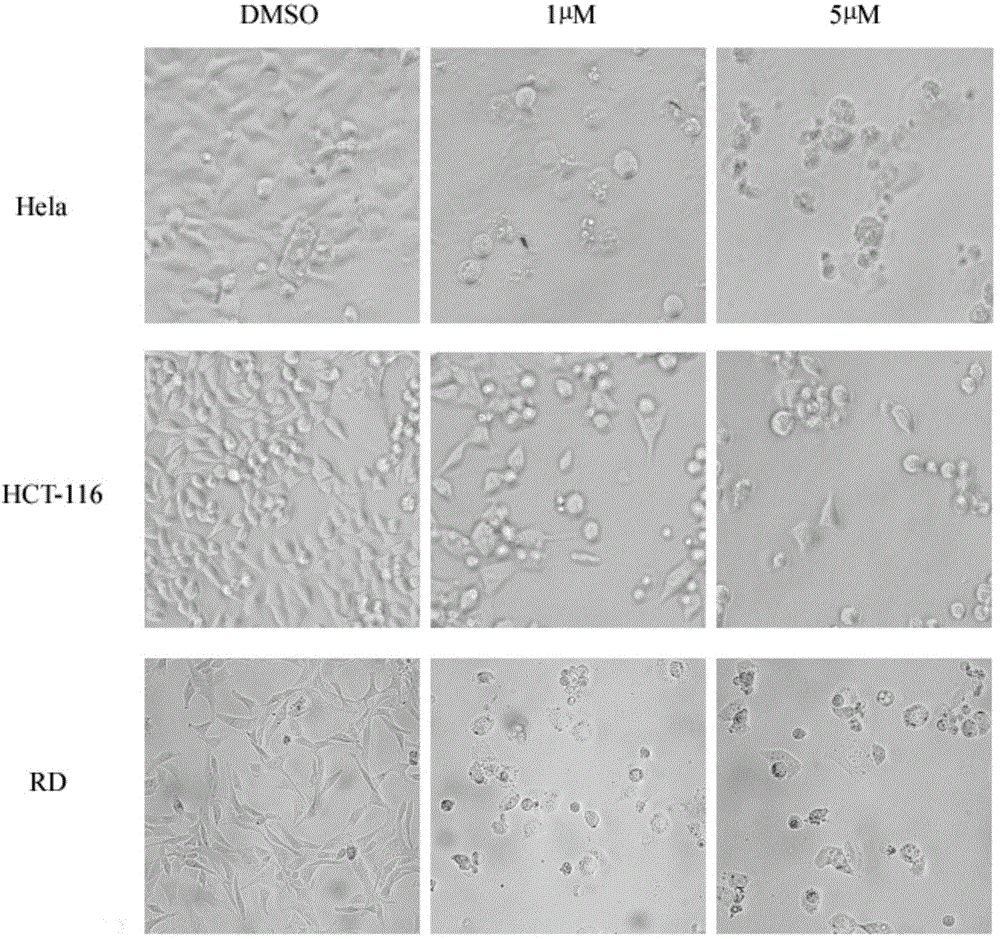
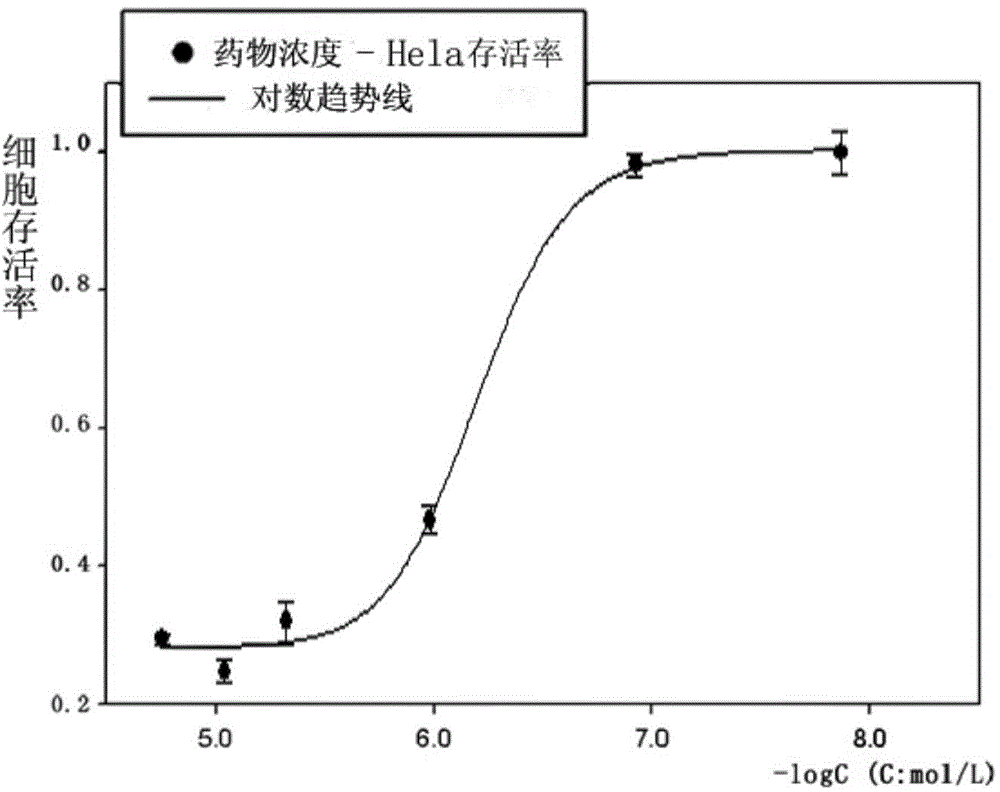
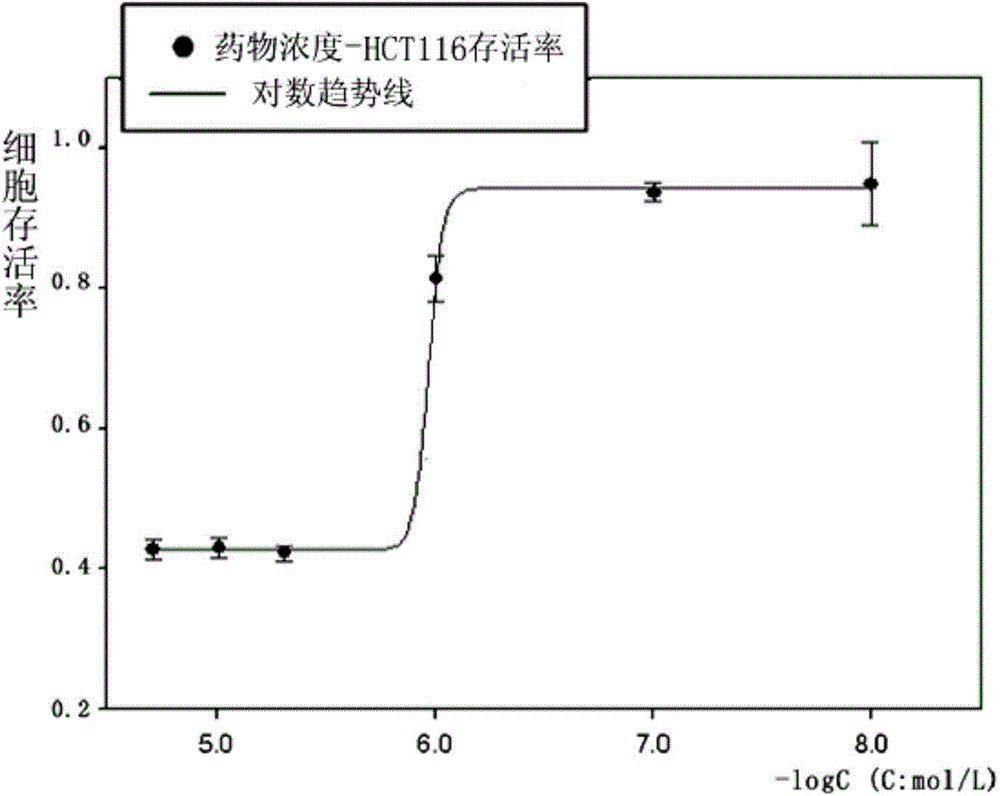
[0041] Example 2: Determination of the half inhibitory concentration of 2-(4-hydroxyphenylimino)-5-(3-methoxybenzylidene)-4-thiazolidinone on cancer cell growth by SRB method
[0042] 5% CO at 37°C 2 Three cancer cells, Hela, HCT-116, and RD, were cultured in DMEM / 10% fetal bovine serum medium under the condition of . Three kinds of cancer cells in the logarithmic phase were collected and seeded in 96-well plates respectively, and incubated for 24 hours. Add different concentrations (20, 10, 5, 1, 0.1, 0.01 μM) of 2-(4-hydroxyphenylimino)-5-(3-methoxybenzylidene)-4-thiazolidinone ( Compound I) After incubation for 48 hours, 50 μL of trichloroacetic acid solution (30%) was added and fixed at 4°C for one hour. Shake off the solution, rinse with high-purity water five times, add 100 μL sulforhodamine B to stain for 30 minutes after drying, rinse with 1% acetic acid solution five times after drying. After drying, add 100 μL of Tris solution to fully dissolve, and measure the ab...
Embodiment 3
[0043] Example 3: Determination of the effect of 2-(4-hydroxyphenylimino)-5-(3-methoxybenzylidene)-4-thiazolidinone on cancer cell cycle by PI staining flow cytometry
[0044] Hela, HCT-116, and RD cancer cells in the logarithmic growth phase were inoculated into 6 cm culture dishes at an amount of 200,000 cells per 3 mL of medium volume. After culturing for 24 hours, each cancer cell group was treated with DMSO and 5.0 μM 2-(4-hydroxyphenylimino)-5-(3-methoxybenzylidene)-4-thiazolidinone (Compound I) deal with. After incubation for 24 h, the cells were digested on ice, and the cell suspension was collected, washed twice with PBS after centrifugation. Add 10 mL of 70% ice ethanol to suspend at -20°C overnight. Centrifuge the cell suspension, wash twice with PBS, add 500 μL PI / 5 μL RNAaseA to the cell mass and stain at 4°C for half an hour in the dark. The DMSO group was used as a negative control, and the cell cycle was detected by flow cytometry with standard procedures. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com