Method for recovering furan ammonium salt from furan ammonium salt waste residue
A technology of furan ammonium salt and waste residue liquid, which is applied in the direction of organic chemistry, can solve the problem of low production cost, and achieve the effects of low production cost, good quality and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
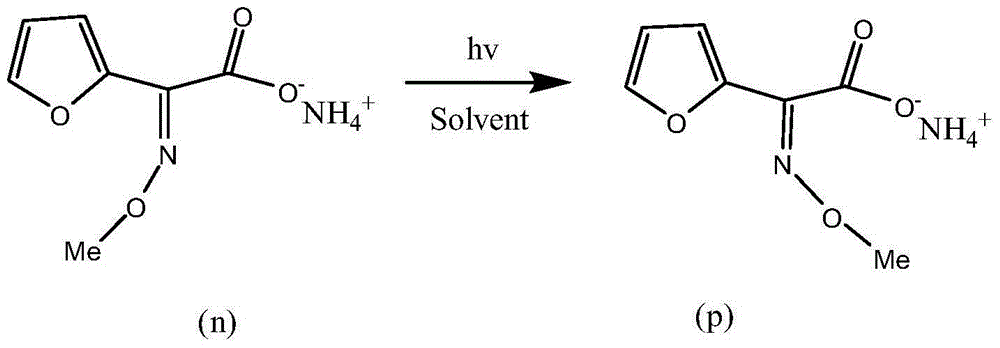
[0023] 500g furan ammonium salt waste residue liquid (E / Z=90:10), diluted with 3000ml water, was added to a 5L photochemical reactor. Utilize 313nm ultraviolet light generated by a high-pressure mercury lamp of 500W to irradiate for 3 hours while stirring, and the temperature in the reactor is controlled at 50°C. The reaction solution obtained after the reaction was detected by HPLC, E / Z=55 / 45. The reaction solution was processed by a known separation method to obtain 195 g of the furan ammonium salt product, and the product yield was 39%.
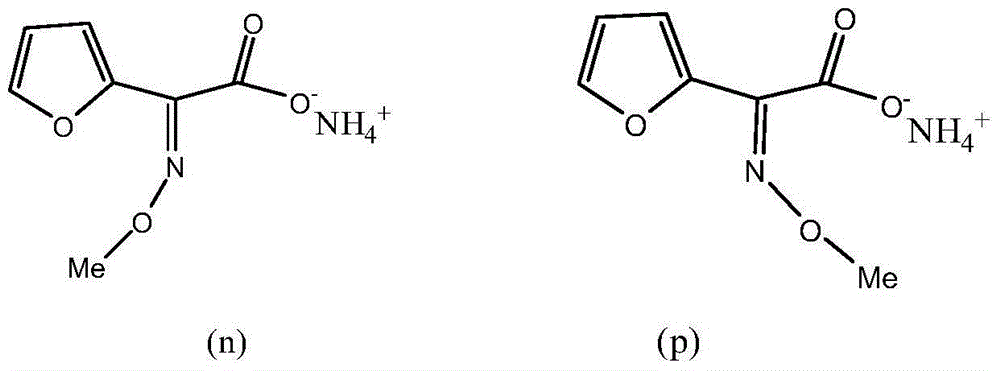
[0024] The furan ammonium salt product described here refers to the chemical name (Z)-2-methoxyimino-2-(furan-2-yl)ammonium acetate, (alias, methoxyiminofuran ammonium acetate;( Z) ammonium furyl salt; the English name is Syn-2-Methoxyimino-2-(2-Furyl)-Acetic Acid-Ammonia Salt) product.
Embodiment 2-11
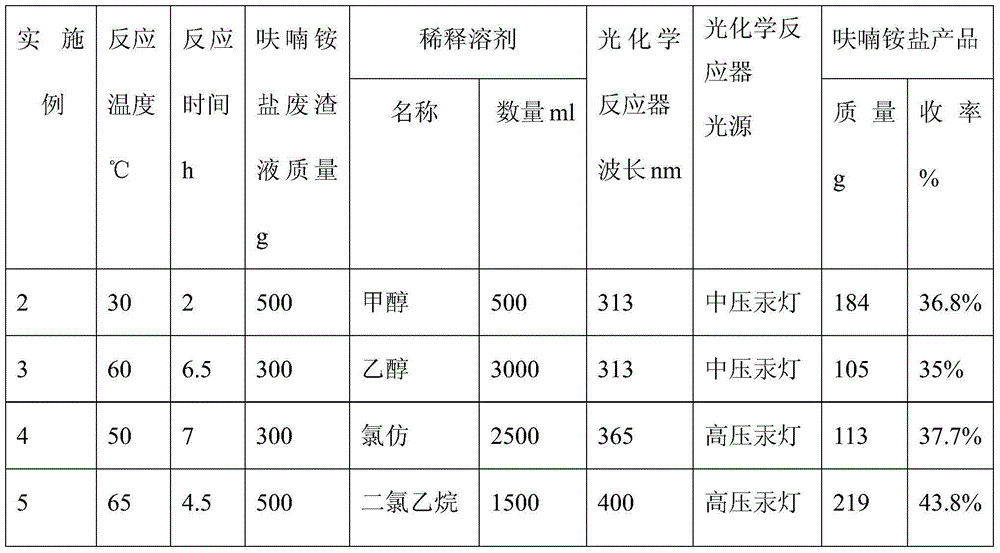
[0026] The treatment process of ammonium furan salt waste residue liquid is completely with embodiment 1 among the embodiment 2-11. Embodiment 2-11 was completed by changing the type and amount of diluting solvent used, photochemical reactor light source and wavelength, reaction time, reaction temperature, quality of furan ammonium salt waste residue liquid and other variables. The specific test data are shown in Table 1.
[0027] The test data of table 1 embodiment 2-11
[0028]
[0029]
[0030] The product that embodiment 1-11 obtains is done quality inspection, and result is as follows:
[0031] The product appearance that embodiment 1-11 obtains is all white crystalline powder;
[0032] Identification (HPLC): the products that embodiment 1-11 obtains all meet the requirements;
[0033] Content (HPLC): the products obtained in Examples 1-11 are all ≥ 99.5%;
[0034] Moisture (K.F): the products obtained in Examples 1-11 are all ≤0.20%;
[0035] Absorbance: the p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com