Diheterocyclic compound as well as synthesis method and application thereof
A synthesis method and compound technology, applied in the fields of botanical equipment and methods, application, organic chemistry, etc., can solve the problem of less research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
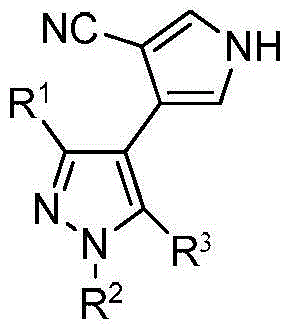
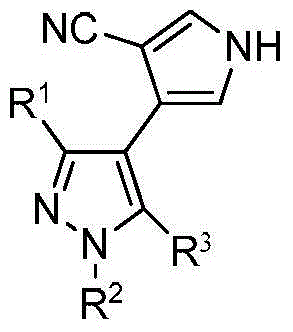
[0019] Specific embodiment 1: The general structural formula (I) of the biheterocyclic compound in this embodiment is:
[0020]
[0021] where R 1 is methyl or trifluoromethyl; R 2 is methyl or phenyl; R 3 It is a phenoxy group, a para-substituted phenoxy group or a chlorine atom, and the para-substituent in the para-substituted phenoxy group is a halogen, an alkyl group with 1 to 3 carbon atoms, an alkoxy group or a tert-butyl group .
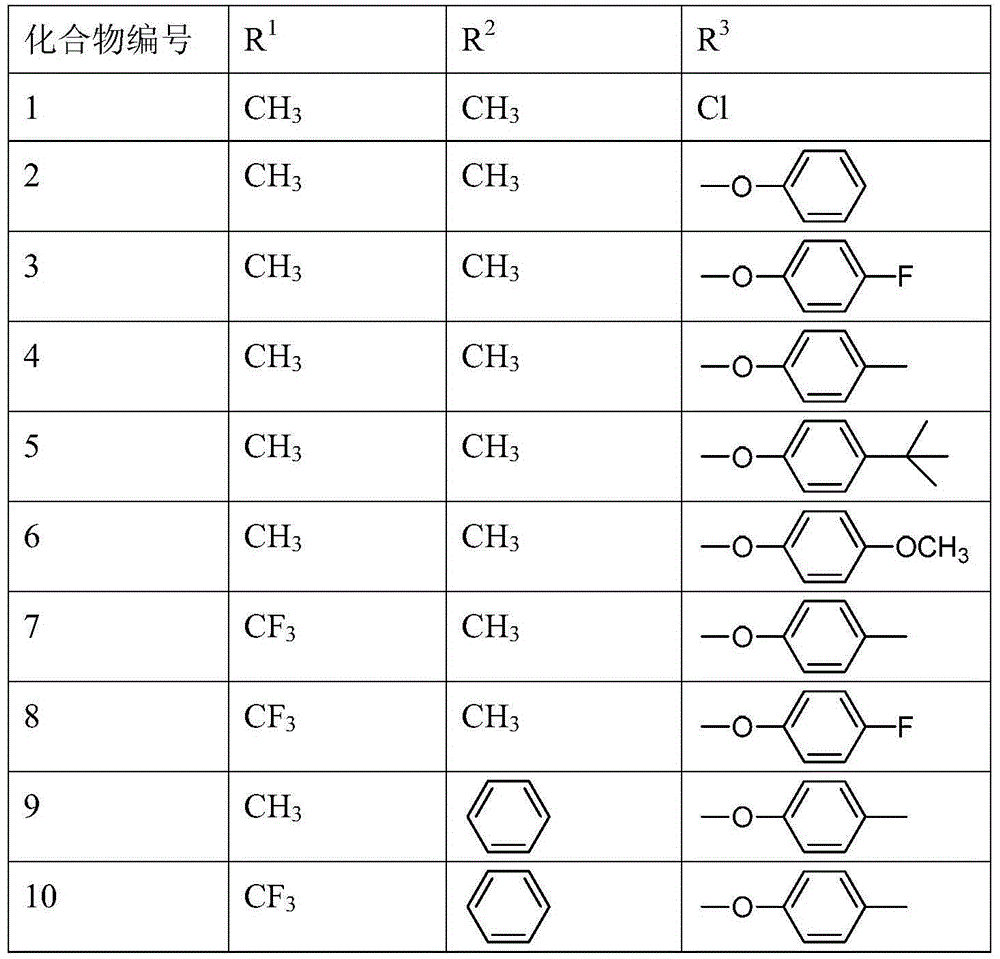
[0022] The following table is an example, listing 10 biheterocyclic compound compounds with the general formula (I), but the present invention is not limited to the compounds in the following table.
[0023]
specific Embodiment approach 2
[0024] Specific embodiment two: The biheterocyclic compound in this embodiment is 4-(5-chloro-1,3-dimethyl-1H-pyrazol-4-yl)-1H-pyrrole-3-carbonitrile, 4-(5 -(4-fluorophenoxy)-1,3-dimethyl-1H-pyrazol-4-yl)-1H-pyrrole-3-carbonitrile, 4-(4-(tert-butyl)phenoxy) -1,3-Dimethyl-1H-pyrazol-4-yl)-1H-pyrrole-3-carbonitrile, 4-(5-(4-methylphenoxy)-1-methyl-3-( Trifluoromethyl)-1H-pyrazol-4-yl)-1H-pyrrole-3-carbonitrile or 4-(5-(4-fluorophenoxy)-1-methyl-3-(trifluoromethyl )-1H-pyrazol-4-yl)-1H-pyrrole-3-carbonitrile.
specific Embodiment approach 3
[0025] Specific embodiment three: the synthetic method of the biheterocyclic compound of the present embodiment is implemented according to the following steps:
[0026] 1. According to the molar ratio of acyl ethyl ester compound and hydrazine compound as 1: (1~1.1), add hydrazine compound dropwise into the reaction bottle containing acyl ethyl ester compound, and control the temperature at 15~20°C through a cold water bath. React under the condition of ℃ for 1-2 hours, then use vacuum distillation for 2.5-3.5 hours, then add toluene to the reaction system to reflux and divide the water, after removing water, cool down to precipitate the solid phase, and the solid phase is filtered and dried After obtaining the 5-pyrazolone compound;
[0027] 2. Add the 5-pyrazolone compound obtained in step 1 into the DMF (N,N-dimethylformamide) solution, add phosphorus oxychloride dropwise at a temperature of 10-20°C, and then raise the temperature to 110- 115°C, react for 5-10 hours, pour...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com