Production of substituted phenylene aromatic diesters
A technology of phenylene dibenzoate and methyl catechol, which is applied in the field of preparation of phenylene aromatic diesters, can solve problems such as difficult to obtain, unreliable, and limited commercial supply, and achieve simplified preparation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
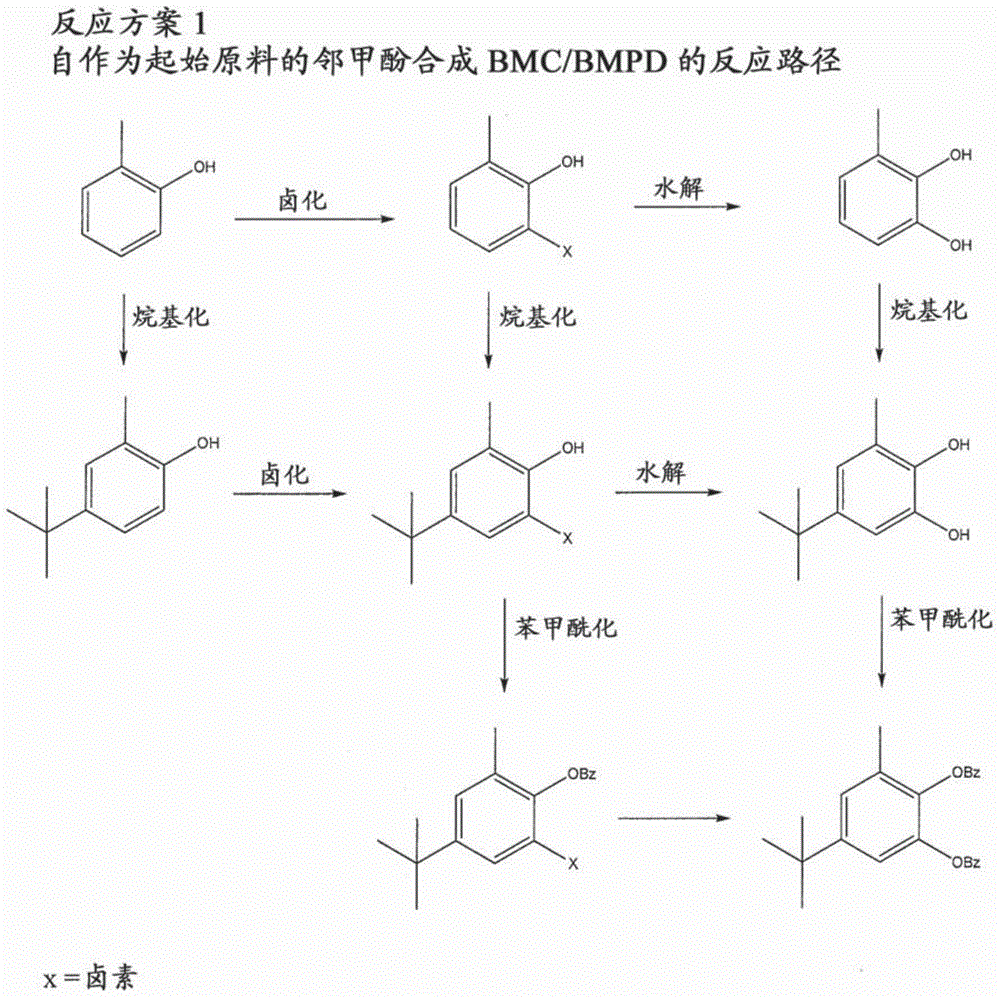
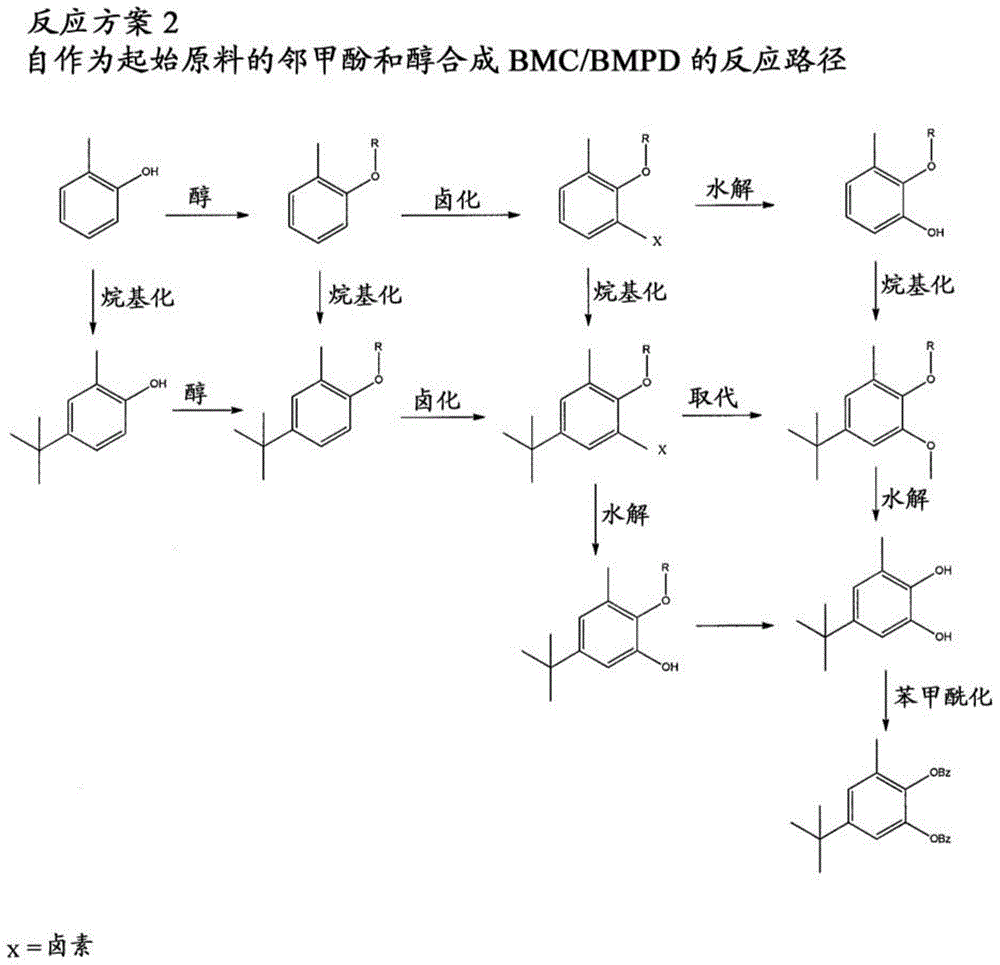
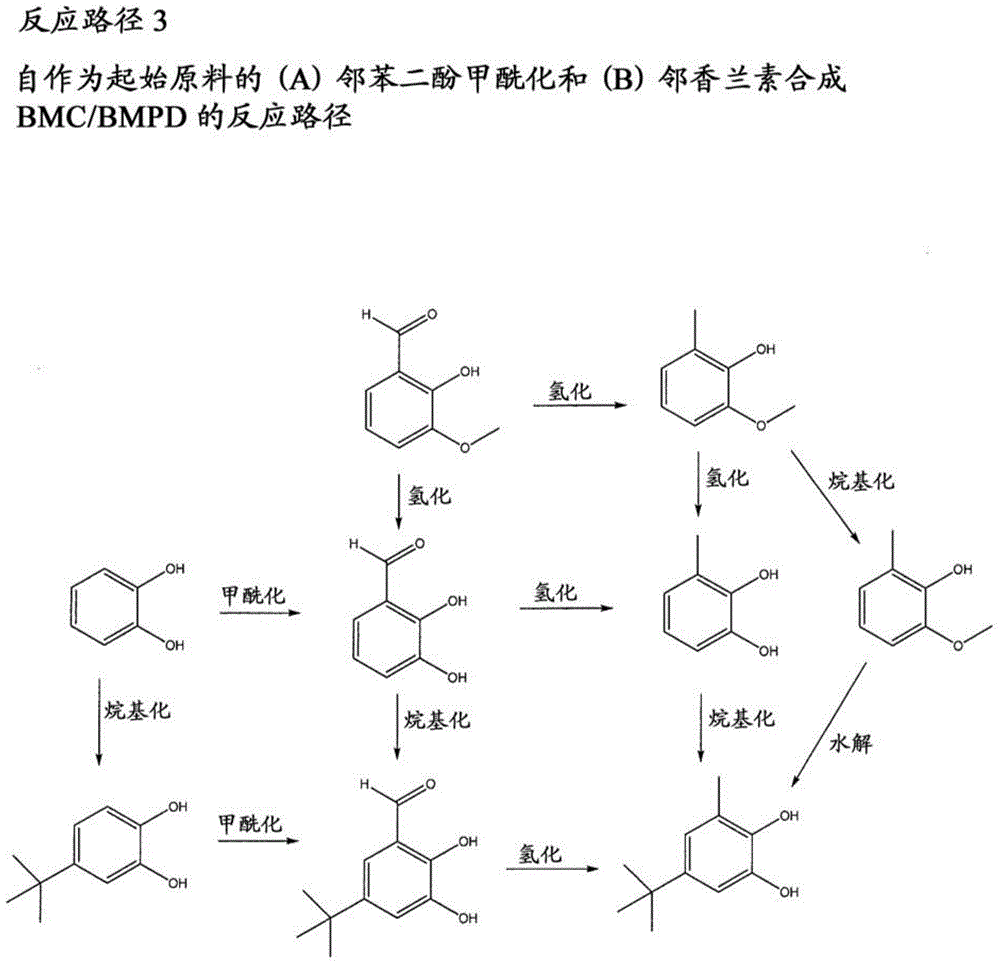
[0027] The present disclosure relates to the preparation of substituted phenylene aromatic diesters. The compound 5-tert-butyl-3-methylcatechol (or "BMC") has been found to be useful in the preparation of substituted phenylene aromatic diesters, 5-tert-butyl-3-methyl-1, A potent precursor of 2-phenylene dibenzoate (or "BMPD"). BMPDs are efficient internal electron donors in Ziegler-Natta catalysts. The methods disclosed in the present invention advantageously provide an economical (time, energy, productivity, and / or starting reagent economy), simple, scalable synthetic route to BMCs with yields comparable to its Acceptable for commercial / industrial applications. Thus, the reliable preparation of BMC contributes to the reliable and economical preparation of 5-tert-butyl-3-methyl-1,2-phenylene dibenzoate (BMPD), which in turn contributes to Olefin-based polymers, especially propylene-based polymers, are prepared with improved properties.
[0028] 1. Preparation of BMC / BMPD f...
Embodiment
[0109] Preparation of 2-methoxy-6-methylphenol by hydrogenation of o-vanillin
[0110] Due to the use of hydrogen gas, the reaction was carried out in a drybox for safety measures. During the procedure, the moisture-proof box was periodically purged with nitrogen to ensure that there was no accumulation of hydrogen. An adapter with a balloon on one end was attached to a 250 mL flask with a side arm and a magnetic stir bar. 1 g of Pd on carbon (5% Pd) was slowly added to the flask. Subsequently, 7.6 g of o-vanillin and 100 ml of methanol were added. Hydrogen was introduced into the flask system through the side arm until the balloon inflated to a volume of approximately 250 ml. The reaction was stirred at room temperature for 3 days. Hydrogen is added as the balloon shrinks due to reaction and diffusion. GC samples were taken to monitor the reaction. When completion of the reaction was evidenced by the appearance of intermediates first followed by products, the gas in the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com