Method for preparing carbazole and vinyl carbazole
A technology of carbazole and carbazole derivatives is applied in the field of preparation of carbazole and its derivatives, and can solve the problems of high production cost and low purity of carbazole
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
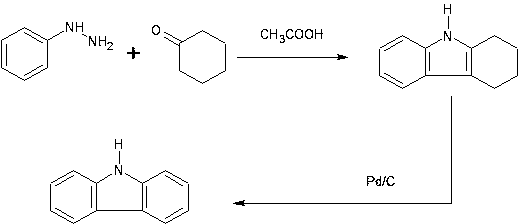
[0051] Mix 150g of cyclohexanone and 550g of acetic acid, heat to reflux state (external temperature 160°C, internal temperature 122°C), add dropwise 180g of phenylhydrazine, complete the dropwise addition within 2 hours, and proceed to the reaction.
[0052] After 1.5h, the reaction was completed, and solids were precipitated after cooling, filtered, and the filter cake was washed with water and 70% ethanol to obtain 195g of intermediate A-H (R 1 and R 2 Both are H).
[0053] Add 100g of intermediate A-H, 2g of Pd / C (water content 54%, catalyst content 10%) into 300ml n-octanol, and suspend. Heating to 164~174°C (external temperature 180°C) for reaction, after 28 hours the reaction was completed, cooled to 0°C, filtered, washed with methanol, and dried to obtain 71.7g of carbazole.
Embodiment 2
[0055] Mix 300g of cyclohexanone and 1100g of acetic acid, heat to reflux state (external temperature 130°C, internal temperature 120°C), add dropwise 350g of phenylhydrazine, complete the dropwise addition within 3 hours, and proceed to the reaction.
[0056] After 1 h, the reaction was completed, and solid precipitated after cooling, filtered, washed the filter cake with water and 75% ethanol, and recrystallized from methanol to obtain 408 g of intermediate A-H.
[0057] 408g of intermediate A-H, 17g of Pd / C (54% water content, 10% catalyst content) were added to 1100ml n-octanol, and suspended. Heat to 145~177°C (external temperature 180°C) for reaction, after 24 hours the reaction is complete, cool to 0°C, filter, wash with acetone, and dry to obtain 330g of carbazole.
[0058] The products obtained in the above examples were detected by nuclear magnetic resonance and infrared spectroscopy, and all of them obtained the target products.
Embodiment 3
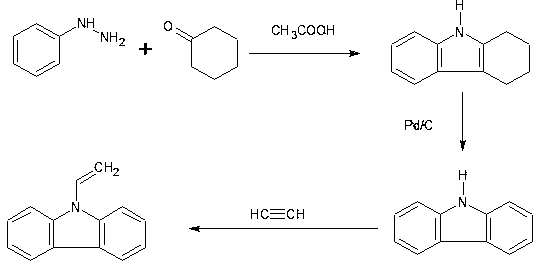
[0060] The carbazole prepared in the foregoing examples is a raw material, and the method for preparing vinyl carbazole is as follows:
[0061] 20g carbazole, 100ml N-methylpyrrolidone, 1.2g potassium hydroxide, pass through acetylene to 0.2Mpa, hold the pressure for 30min, raise the temperature to 140°C, react for 3.5h, cool down and sample for LC analysis, the reaction is complete, methanol recrystallized to get Vinyl carbazole, LCMS 99.5% purity by liquid chromatography mass spectrometry analysis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com