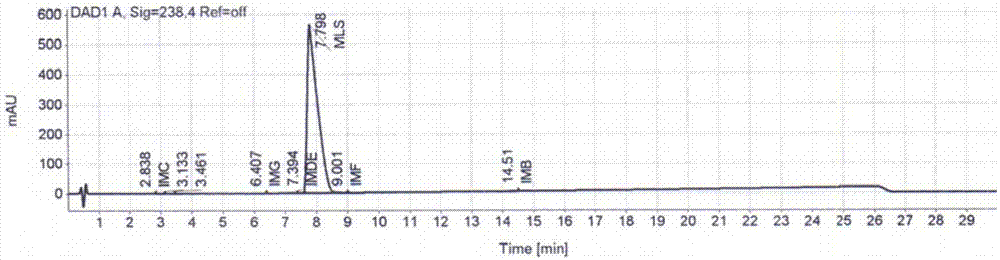
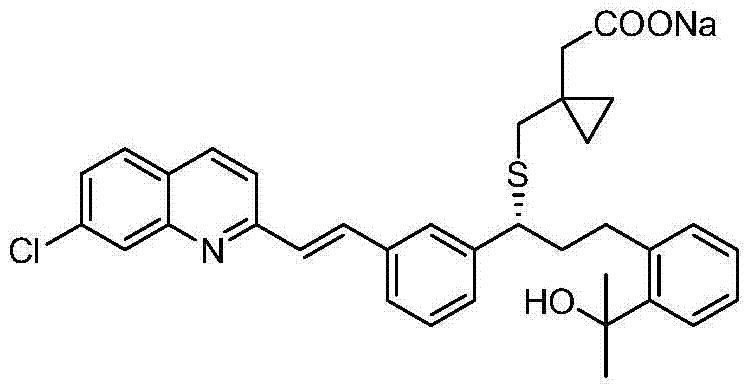
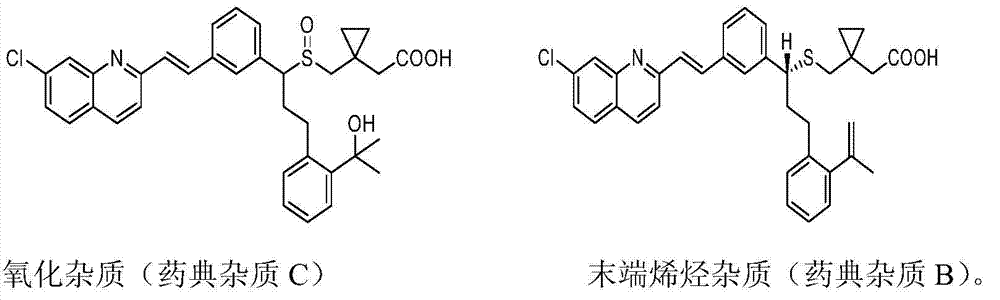
Preparation method of montelukast sodium
A technology of montelukast sodium and hexylamine salt, which is applied in the field of preparation of montelukast sodium salt, can solve the problems of low impurity removal efficiency and complicated operation, and achieve high product purity, simple operation and high yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Put 20g (0.034 moles) of montelukast acid into a 500ml three-necked bottle, add 200ml of toluene, replace with nitrogen twice, add 7.45ml (0.0374 moles) of dicyclohexylamine, stir until the material solution is clear, add seed crystals, and keep at 25°C Stir for 24 hours, and filter to obtain 30 g of montelukast dicyclohexylamine salt wet material.
[0031] Add the above-mentioned montelukast dicyclohexylamine salt into 200ml ethyl acetate, replace with nitrogen twice, add 100ml of 2.5% glacial acetic acid aqueous solution, stir for 30 minutes, let stand, and separate to remove the water layer; wash the organic phase once with 100ml of water, separate Remove the water layer. Dry the organic phase with magnesium sulfate, filter, and evaporate the solvent; add 200ml of toluene, stir evenly, replace with nitrogen twice, add 5.11ml of di-n-propylamine (0.0374 moles), stir until the material solution is clear, add seed crystals, and stir at 25°C 24h. Filter and dry. 20 g ...
Embodiment 2
[0034] Put 20g (0.034 moles) of montelukast acid into a 500ml three-necked bottle, add 100ml of acetone, replace nitrogen twice, add 10.15ml (0.051 moles) of dicyclohexylamine, stir until the material solution is clear, add seed crystals, and heat at 35°C Stir for 20 hours, and filter to obtain 30 g of montelukast dicyclohexylamine salt wet material.
[0035] Add the above-mentioned montelukast dicyclohexylamine salt into 200ml of ethyl acetate, replace with nitrogen twice, add 100ml of 0.5M tartaric acid aqueous solution, stir for 30 minutes, let stand, and separate to remove the water layer; wash the organic phase once with 100ml of water, separate Remove the water layer. Dry the organic phase with anhydrous sodium sulfate, filter, and evaporate the solvent; add 200ml of toluene, stir evenly, replace with nitrogen twice, add 5.11ml (0.0374 moles) of di-n-propylamine, stir until the material liquid is dissolved, add seed crystals, 20 Stir at ℃ for 20h. After filtering and d...
Embodiment 3
[0038] Put 20g (0.034 moles) of montelukast acid into a 500ml three-necked bottle, add 200ml of toluene, replace with nitrogen twice, add 7.45ml (0.0374 moles) of dicyclohexylamine, stir until the material solution is clear, add seed crystals, and keep at 25°C Stir for 24 hours, and filter to obtain 30 g of montelukast dicyclohexylamine salt wet material.
[0039]Add the above-mentioned montelukast dicyclohexylamine salt into 200ml of dichloromethane, replace with nitrogen twice, add 100ml of 2.5% glacial acetic acid aqueous solution, stir for 30 minutes, let stand, and separate to remove the water layer; wash the organic phase once with 100ml of water, separate Remove the water layer. Dry the organic phase with magnesium sulfate, filter, and evaporate the solvent; add 200ml of toluene, stir evenly, replace with nitrogen twice, add 5.11ml of di-n-propylamine (0.0374 moles), stir until the material solution is clear, add seed crystals, and stir at 25°C 24h. Filter and dry. 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com