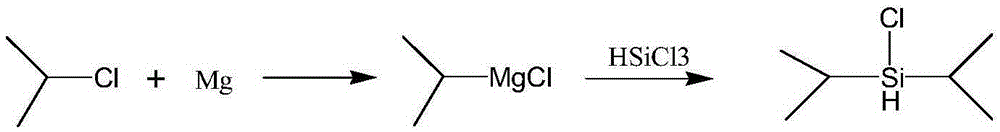
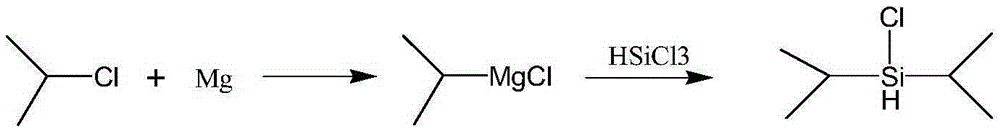
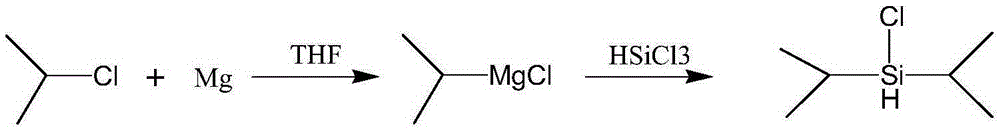
A kind of synthetic method of diisopropyl chlorosilane
A technology of diisopropyl chlorosilane and synthesis method, which is applied in chemical instruments and methods, compounds of elements of Group 4/14 of the periodic table, organic chemistry, etc. Problems such as the supply of kilograms and above, which are not conducive to industrial scale-up production, achieve the effect of reasonable selection and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] to N 2 Add 5.10 g (0.21 mol) of magnesium chips and 15 mL of tetrahydrofuran into a 500 mL four-necked flask under protection, and add 2-chloropropane (16.81 g, 0.214 mol) into the constant dropping funnel. Quickly add about 4 mL of 2-chloropropane (about one-fifth of the total 2-chloropropane) dropwise into a 500 mL four-necked flask through a constant dropping funnel, and dilute the remaining 2-chloropropane with 90 mL of tetrahydrofuran. Heat to 40-65°C to initiate the reaction. After initiation, add the remaining 2-chloropropane solution in tetrahydrofuran dropwise, and keep the temperature at 40-65°C. After the dropwise addition, keep warm at 40-65°C for 0.5-1h, then cool to 20-30°C. A mixed solution of 13.68g (0.1mol) trichlorosilane and 41.04g n-hexane was added dropwise, the system exothermed violently and produced a large amount of white solid, the temperature was controlled at 20-30°C. After dropping, react for 0.5-1h, filter with suction, wash the filter ca...
Embodiment 2
[0026] to N 2 Add 5.10 g (0.21 mol) of magnesium chips and 15 mL of tetrahydrofuran into a 500 mL four-necked flask under protection, and add 2-chloropropane (16.81 g, 0.214 mol) into the constant dropping funnel. Quickly add about 4 mL of 2-chloropropane (about one-fifth of the total 2-chloropropane) dropwise into a 500 mL four-necked flask through a constant dropping funnel, and dilute the remaining 2-chloropropane with 90 mL of tetrahydrofuran. Heat to 40-65°C to initiate the reaction. After initiation, add the remaining 2-chloropropane solution in tetrahydrofuran dropwise, and keep the temperature at 40-65°C. After the dropwise addition, keep warm at 40-65°C for 0.5-1h, then cool to 20-30°C. A mixed solution of 13.68g (0.1mol) trichlorosilane and 41.04g n-hexane was added dropwise, the system exothermed violently and a large amount of white solids were produced, the temperature was controlled at 10-20°C. After dropping, react for 0.5-1h, filter with suction, wash the fil...
Embodiment 3
[0028] to N 2 Add 5.10 g (0.21 mol) of magnesium chips and 15 mL of tetrahydrofuran into a 500 mL four-necked flask under protection, and add 2-chloropropane (16.81 g, 0.214 mol) into the constant dropping funnel. Quickly add about 4 mL of 2-chloropropane (about one-fifth of the total 2-chloropropane) dropwise into a 500 mL four-necked flask through a constant dropping funnel, and dilute the remaining 2-chloropropane with 90 mL of tetrahydrofuran. Heat to 40-65°C to initiate the reaction. After initiation, add the remaining 2-chloropropane solution in tetrahydrofuran dropwise, and keep the temperature at 40-65°C. After the dropwise addition, keep warm at 40-65°C for 0.5-1h, then cool to 20-30°C. A mixed solution of 13.68g (0.1mol) of trichlorosilane and 41.04g of n-hexane was added dropwise, the system exothermed violently and a large amount of white solids were produced, and the temperature was controlled at 0-10°C. After dropping, react for 0.5-1h, filter with suction, was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com