Synthesis method and application of flexible carboxylic acid and transition metal complex
A technology of transition metal and synthesis method, which is applied in the application field of catalyzing the hydroxylation of phenol, and can solve the problems of less research on flexible carboxylic acid ligands and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] (1) Add 95wt% ethanol to the reaction kettle containing the organic ligand 5-amino-1,2,4-triazole-3-carboxylic acid to dissolve completely,
[0025] (2) Prepare an aqueous solution of lead tetrafluoroborate with a concentration of 50wt%,
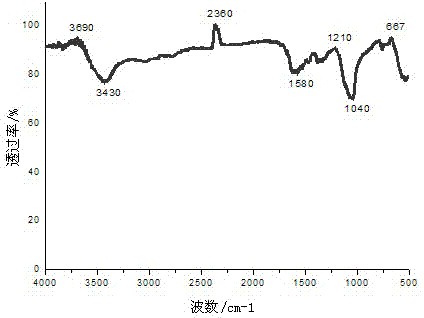
[0026] (3) By solvothermal method, according to the molar ratio of metal salt to organic ligand 1:1, add the metal salt solution to the organic ligand solution obtained in step (1) and mix well, adjust the pH to 3-5, heat to 160°C and Boil at constant temperature for 48 hours, cool down to room temperature, wash and filter to obtain colorless needle-like crystals, labeled as complex 1. The infrared spectrum of the complex is shown in figure 1 shown by figure 1 It can be seen that 3430 cm -1 Absorption peaks appear within the range, indicating that crystal water exists in the complex. at 1720-1680 cm -1 There is no absorption peak in the range, indicating that the carboxyl group COOH has been completely deprotonated.
Embodiment 2
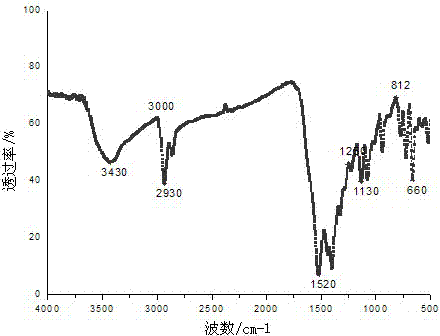
[0028] The organic ligand is adipic acid, the metal salt is lead nitrate, the other processes are the same as in Example 1, and a colorless block crystal is obtained, which is marked as complex 2. The infrared spectrum of the complex is shown in figure 2 As can be seen from the figure, 3430 cm -1 A broad absorption peak appears in the range, indicating that there is coordination water or crystal water in the complex. at 1720-1680 cm -1 There is no absorption peak in the range, indicating that the carboxyl COOH has been completely deprotonated.
Embodiment 3
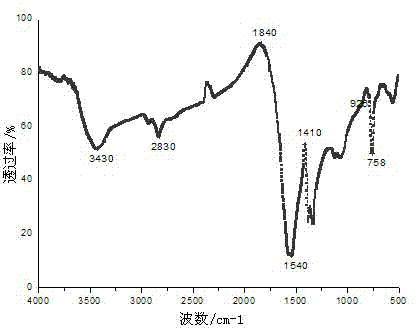
[0030] The organic ligand is 2,3-quinoline dicarboxylic acid, the metal salt is lead nitrate, the other processes are the same as in Example 1, and light yellow needle-like crystals are obtained, which are marked as complex 3. The infrared spectrum of the complex is shown in image 3 Shown, as can be seen from the figure, 3300-3500 cm -1 Absorption peaks appear within the range, indicating that coordination water or crystal water exists in the complex. at 1720-1680 cm -1 There is no absorption peak in the range, indicating that the carboxyl COOH has been completely deprotonated. 1540cm -1 It is C=O stretching vibration.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com