Preparation method of tribenoside isomers
A technology of tribenzyl sugar and isomerization, which is applied in the field of preparation of tribenzyl glycoside isomerization monomer, can solve the problems of low purity, long time of tribenzyl glycoside isomerization monomer, etc., and achieves a simplified and easily controllable process technology. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
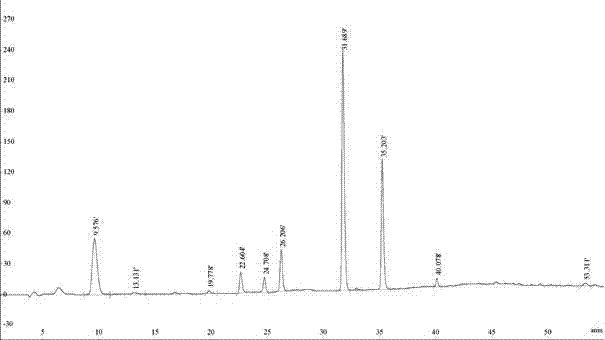
[0016] 1. Take 50 g of crude tribenzyl glucoside and mix it with n-hexane / ethyl acetate solution with a volume ratio of 4:1, configure it into a saturated solution, put it in a filter device, and filter to remove solid particles;
[0017] 2. Inject the tribenzyl glucoside solution into the preparative liquid phase preparative chromatographic separation system, the dynamic axial compression column size is Φ50×250mm, the silica gel filler is the product of Fuji, Japan, the particle size is 20~45um, the sample volume is 30g, and the mobile phase flow rate It is 80ml / min. At the initial stage of separation, the mobile phase n-hexane / ethyl acetate volume ratio ranged from 98 / 2 to 90 / 10. After elution for 40 minutes, the mobile phase n-hexane / ethyl acetate volume ratio was switched to 80 / 20-70 / 30. When the separation time reaches 65 minutes, switch the mobile phase to pure ethyl acetate, so that the impurities at the back end can be washed out, and a separation cycle ends. The dete...
Embodiment 2
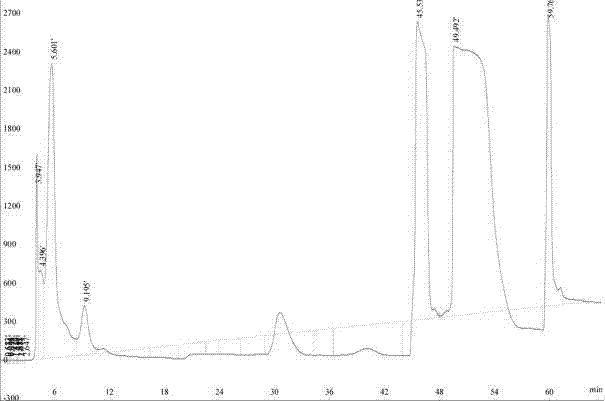
[0019] 1. Take 150g of crude tribenzyl glucoside and mix it with n-hexane / ethyl acetate solution with a volume ratio of 6:1, configure it into a saturated solution, place it in a filter device, and filter to remove solid particles;
[0020] 2. Inject the tribenzyl glucoside solution into the preparative liquid phase preparative chromatographic separation system, the dynamic axial compression column size is Φ100×250mm, the silica gel filler is a product of Fuji, Japan, the particle size is 20~45um, the sample volume is 120g, and the mobile phase flow rate It is 300ml / min. At the initial stage of separation, the mobile phase n-hexane / ethyl acetate volume ratio was 98 / 2~90 / 10, and after 40 minutes of elution, the mobile phase n-hexane / ethyl acetate volume ratio was switched to 85 / 15~70 / 30, and the elution At 65 minutes, the mobile phase was switched to pure ethyl acetate, so that the back-end impurities were washed out, and a separation cycle ended. The detection wavelength of t...
Embodiment 3
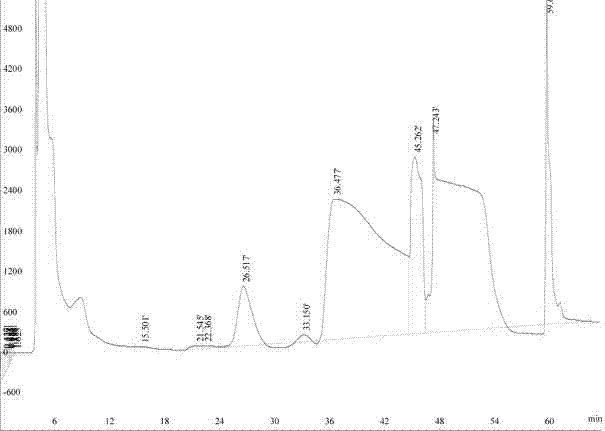
[0022] 1. Take 300g of crude tribenzyl glucoside and mix it with n-hexane / ethyl acetate solution with a volume ratio of 8:1, configure it into a saturated solution, place it in a filter device, and filter to remove solid particles;
[0023] 2. Inject the tribenzyl glucoside solution into the preparative liquid phase preparative chromatographic separation system, the dynamic axial compression column size is Φ150×300mm, the silica gel filler is a product of Fuji, Japan, the particle size is 20~45um, the sample volume is 250g, and the mobile phase flow rate It is 650ml / min. At the initial stage of separation, the mobile phase n-hexane / ethyl acetate volume ratio was 98 / 2~90 / 10, and after 40 minutes of elution, the mobile phase n-hexane / ethyl acetate volume ratio was switched to 85 / 15~70 / 30, and the elution At 65 minutes, the mobile phase was switched to pure ethyl acetate, so that the back-end impurities were washed out, and a separation cycle ended. The detection wavelength of t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com