Synthetic method of betamethasone or prednisolone intermediate
A synthesis method and progesterone technology, which are applied in the production of steroids, bulk chemicals, organic chemistry, etc., can solve the problems of troublesome strain selection, low yield, environmental pollution, etc., and achieve low environmental pollution and operation. Simple, avoid duplication effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
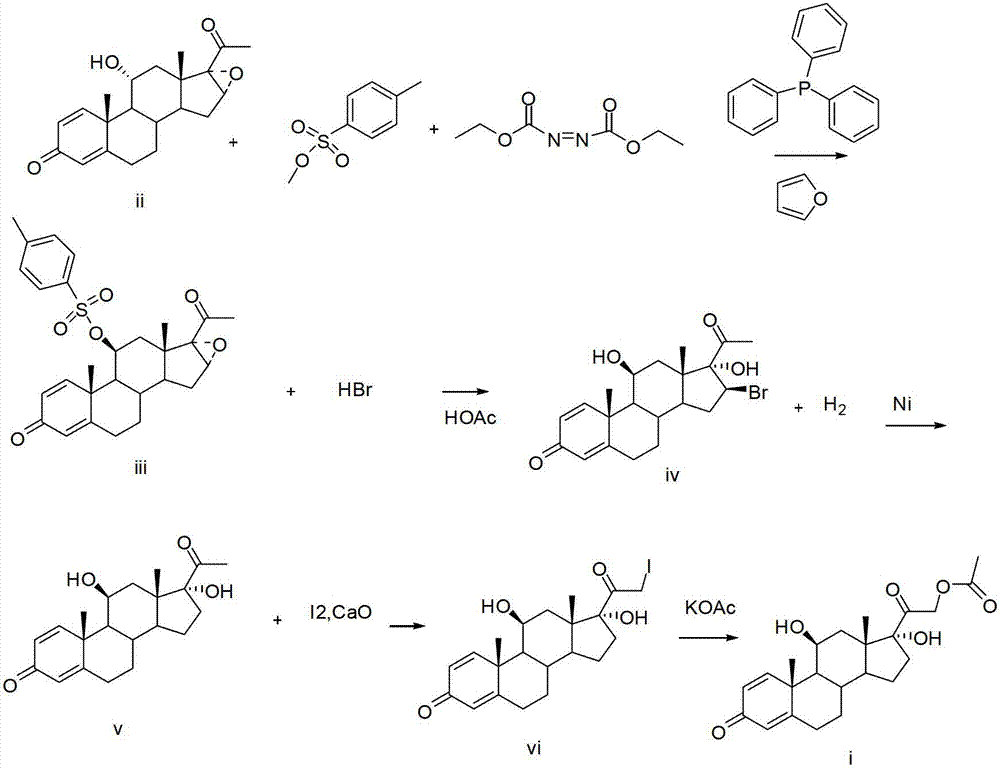
[0029] (a) Add 10g (0.029mol) 11α-hydroxy-1,4-diene-16,17-epoxyprogesterone (ii), about 140ml tetrahydrofuran, 10.1g (0.059mol) Diethyl nitrogen dicarboxylate and 29.9g (0.116mol) triphenylphosphine, stirred for 30 minutes, cooled to -10~-5°C, and started to drop 11.3g (0.061mol) methyl p-toluenesulfonate at -10°C After about 1 hour, the drop was completed, and then the temperature was raised to 20° C., and the reaction was incubated for 2 hours, and the reaction was followed by TLC. Recover tetrahydrofuran from the reaction solution to obtain a solid, add 20ml of methanol, stir for 30 minutes, cool to 0-5°C, filter to obtain 11β-p-toluenesulfonyl-16,17-epoxy-1,4-dienyl progesterone (iii).
[0030] (b) The 11β-p-toluenesulfonyl-16,17-epoxy-1,4-dienyl progesterone (iii) is directly put into another clean 500ml three-necked bottle without drying, and 20ml of glacial acetic acid and 48% HBr 10ml, stirred at 20°C for 3 hours, followed by TLC until the reaction was complete, adde...
Embodiment 2
[0035] (a) Add 10g (0.029mol) 11α-hydroxy-1,4-diene-16,17-epoxyprogesterone (ii), about 140ml tetrahydrofuran, 10.1g (0.059mol) Dipropyl nitrogen dicarboxylate and 29.9g (0.116mol) triphenylphosphine, stirred for 30 minutes, cooled to -10~-5°C, and started to drop 11.3g (0.061mol) methyl p-toluenesulfonate at -10°C After about 1 hour, the drop was completed, and then the temperature was raised to 22° C., and the reaction was incubated for 2.5 hours, and the reaction was followed by TLC. Recover tetrahydrofuran from the reaction solution to obtain a solid, add 20ml of methanol, stir for 30 minutes, cool to 0-5°C, filter to obtain 11β-p-toluenesulfonyl-16,17-epoxy-1,4-dienyl progesterone (iii).
[0036] (b) The 11β-p-toluenesulfonyl-16,17-epoxy-1,4-dienyl progesterone (iii) is directly put into another clean 500ml three-necked bottle without drying, and 20ml of glacial acetic acid and 48% HBr 10ml, stirred at 22°C for 3 hours, followed by TLC until the reaction was complete, a...
Embodiment 3
[0041] (a) Add 10g (0.029mol) 11α-hydroxy-1,4-diene-16,17-epoxyprogesterone (ii), about 140ml toluene, 10.1g (0.059mol) Diethyl nitrogen dicarboxylate and 29.9g (0.116mol) triphenylphosphine, stirred for 30 minutes, cooled to -10~-5°C, and started to drop 11.3g (0.061mol) methyl p-toluenesulfonate at -10°C After about 1 hour, the drop was completed, and then the temperature was raised to 25° C., and the reaction was incubated for 3 hours, and the reaction was followed by TLC. Recover toluene from the reaction solution to obtain a solid, add 20ml of methanol, stir for 30 minutes, cool to 0-5°C, filter to obtain 11β-p-toluenesulfonyl-16,17-epoxy-1,4-dienyl progesterone (iii).
[0042] (b) The 11β-p-toluenesulfonyl-16,17-epoxy-1,4-dienyl progesterone (iii) is directly put into another clean 500ml three-necked bottle without drying, and 20ml of glacial acetic acid and 48% HBr 10ml, stirred at 25°C for 3 hours, followed by TLC until the reaction was complete, added 400ml of ice w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com