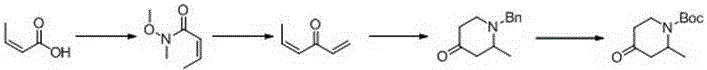A kind of synthetic method of 1-tert-butoxycarbonyl-2-methyl-4-piperidone
A technology of tert-butoxycarbonyl and 1-cbz-2-, which is applied in the field of organic synthesis, can solve the problems of low total yield, danger, and long route, and achieve the effects of easy scale-up production, increased yield, and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The synthetic method of 1-tert-butoxycarbonyl-2-methyl-4-piperidone comprises the following steps:
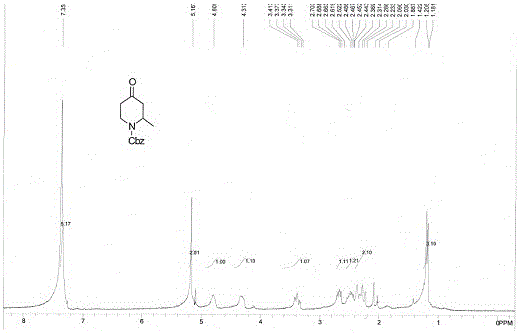
[0044] (1) Dissolve 150 grams of 4-methoxy (1.375 mol) pyridine in 1850 ml of tetrahydrofuran, add 19 ml (1.375 mol) of triethylamine, cool down to minus 40 ° C, and then dropwise add 235 grams (1.375 mol) Cbz-Cl, after the dropwise addition, keep minus 40°C for 20 minutes, under the protection of nitrogen, add dropwise 692ml of ether solution containing 3mol / L methylmagnesium bromide, after the dropwise addition is completed, return to room temperature, and react at room temperature for 2 Hours, TLC, the raw material has reacted completely, quenched the reaction with 500ml of 1Mol / L hydrochloric acid, extracted 3 times with 300 ml of ethyl acetate respectively, combined the organic phases, washed once with saline, dried over sodium sulfate, and evaporated the solvent to obtain reddish brown 323.8 g of the oily substance is 1-Cbz-2-methyl-3,4-dihydro-4-piperidone, and th...
Embodiment 2
[0048] The synthetic method of 1-tert-butoxycarbonyl-2-methyl-4-piperidone comprises the following steps:
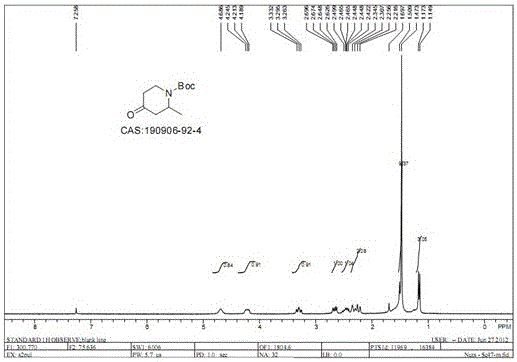
[0049] (1) Dissolve 900 grams of 4-methoxypyridine (8.25mol) in 10 liters of tetrahydrofuran, add 114ml (8.25mol) of triethylamine, cool down to minus 40°C, and then dropwise add 1410 grams (8.25mol) Cbz-Cl, after the dropwise addition, keep minus 40°C for 30 minutes, under the protection of nitrogen, add dropwise 4152ml of ether solution containing 3mol / L methylmagnesium bromide, after the dropwise addition is completed, return to room temperature, and react at room temperature for 2 Hours, TLC, the raw material has reacted completely, quenched the reaction with 2500ml of 1Mol / L hydrochloric acid, extracted 3 times with 1500ml of ethyl acetate respectively, combined the organic phases, washed once with saline, dried over sodium sulfate, and evaporated the solvent to obtain reddish brown 1950 g of the oily substance is 1-Cbz-2-methyl-3,4-dihydro-4-piperidone, and the yie...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com