Application of miR181s in preparing drug for treating acute enteritis
A technology of mir181s and 1.mir181s, applied in the field of biomedical materials, can solve the problem that the understanding of the mechanism of miRNA inhibiting translation is not completely clear, and achieve the effects of low immunogenicity, simple production and synthesis process, and low cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
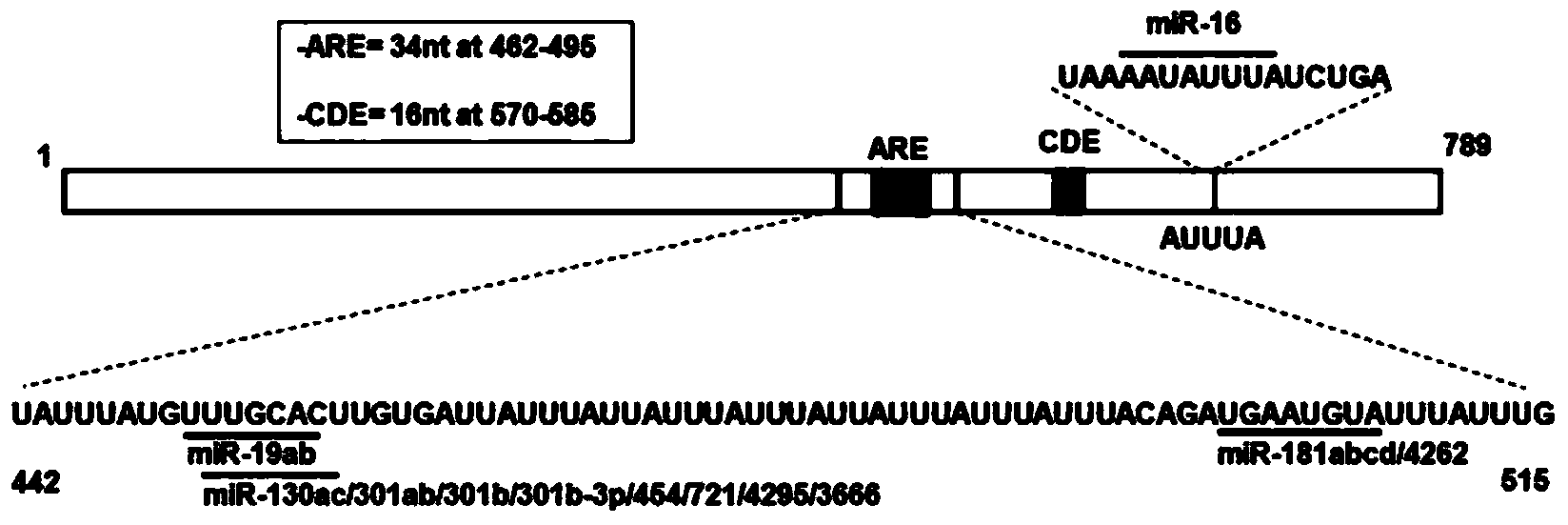
[0033] Example 1: miR181 binds to TNF-α3'-UTR and promotes the degradation of TNF-α mRNA
[0034] 1.1 Experimental method
[0035] 1.1.1 Construction of TNF-α3'-UTR full-length and its point mutation reporter plasmid
[0036] The full-length 3'-UTR target fragment of TNF-α was amplified from cDNA by PCR method, and the full-length fragment was used as a template to design primers to amplify the 3'-UTR fragment of miR-181 binding site mutation.
[0037] PCR amplification primers are as follows
[0038]
[0039] 1.1.2 Co-transfection of cells with plasmid and miRNA fragments and detection of reporter genes
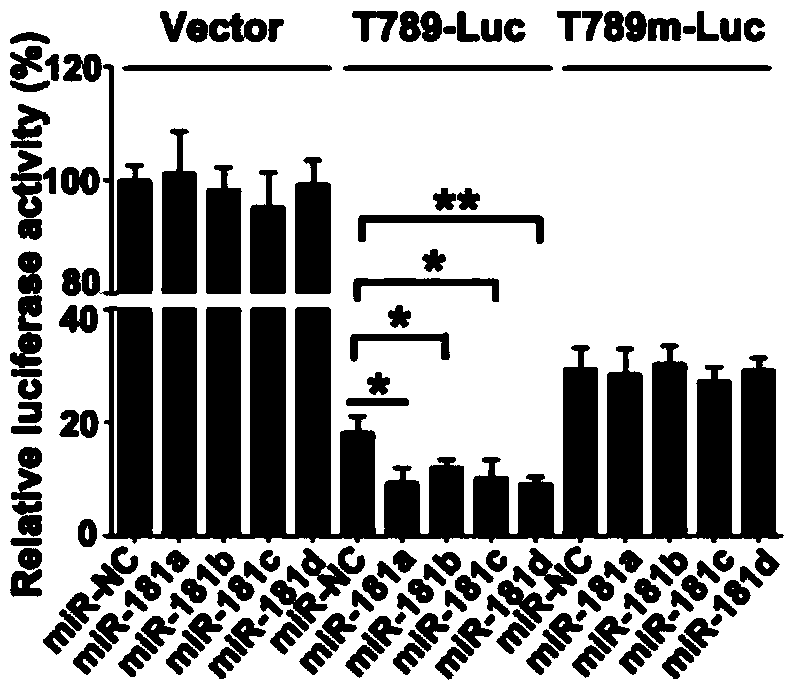
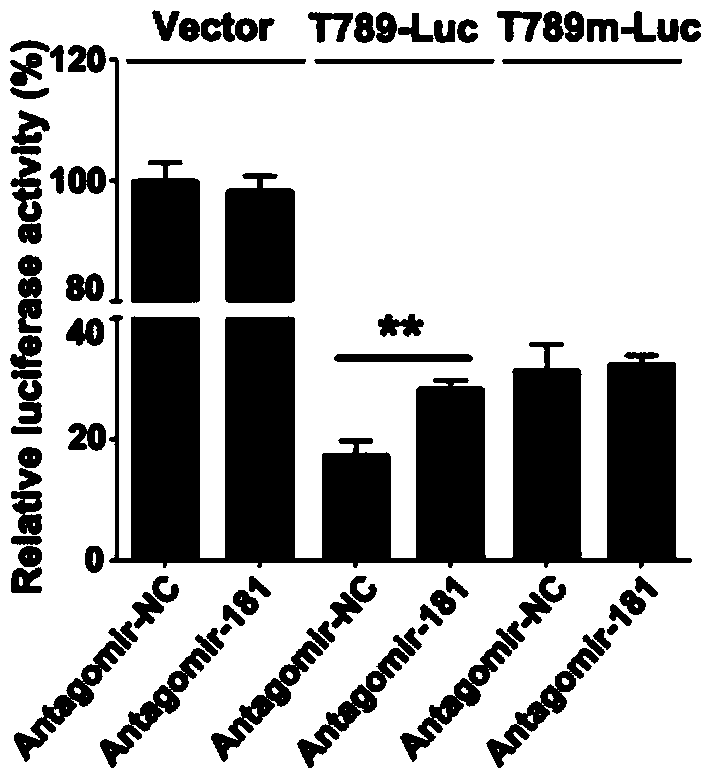
[0040] The PCR products of T789 and T789 mutation obtained above were constructed into pGL3-basic plasmid (purchased by Invitrogen) by molecular cloning method. A549 cells were inoculated in a 24-well plate, and when the cell confluency reached 60%, each well was transfected with the above-mentioned 0.5 μg T789 or T789 mutant luciferase reporter plasmid, 0.05 μg pRL pl...
Embodiment 2
[0054] Example 2: In vivo distribution of cholesterol-modified miR181d
[0055] 2.1 Experimental method
[0056] 2.1.1 Modified miR‐181d (agomir‐181d)
[0057] miRNA is modified by cholesterol, thio, and 2-methoxy (called agomir, mainly used in animal experiments), the stability in vivo is increased, and it can be well absorbed by tissue cells without transfection reagents. In order to facilitate the study of the absorption and distribution of agomir-181d in mice, we introduced Cy5 fluorescent modification on the synthetic fragment ( Figure 6 ).
[0058] 2.1.2 Reverse transcription of miRNA (Stem‐loop method)
[0059] (1) Dilute the RNA to 5.5ng / μl with RNase free water.
[0060] (2) The reaction system is as follows:
[0061]
[0062] The reaction steps are as follows: 16°C for 10 minutes, 42°C for 40 minutes, 85°C for 2 minutes, 4°C forever
[0063] 2.2.3 qPCR of miRNA (Stem‐loop method)
[0064] (1) Dilute the cDNA template 20-100 times with DDW.
[0065] (2) Th...
Embodiment 3
[0078] Example 3: Anti-acute inflammatory bowel disease of cholesterol-modified miR181d
[0079] 3.1 Experimental method:
[0080]3.1.1 Modeling of TNBS-induced colitis in mice
[0081] BALB / c female mice aged 6-8 weeks were selected for the experiment, and the mice were anesthetized by intraperitoneal injection of sodium pentobarbital at a weight of 0.01ml / g. Draw out 100 μl of 2.5% TNBS with a syringe connected to an enema needle, carefully insert the needle into the anus of the mouse to a depth of 3‐4 cm, avoid hurting the intestinal wall during the process, and slowly push in the liquid medicine. The control group was given the same volume of 50% alcohol solution, and the needle was pulled out slowly after the enema was completed. In order to ensure that the sensitizing solution can fully interact with the intestinal tract, the mouse was fixed head down on a Stand on an inclined plane for 30 minutes. Keep the body temperature of the mouse, and put the mouse back into ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com