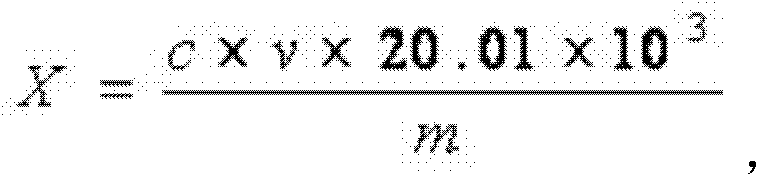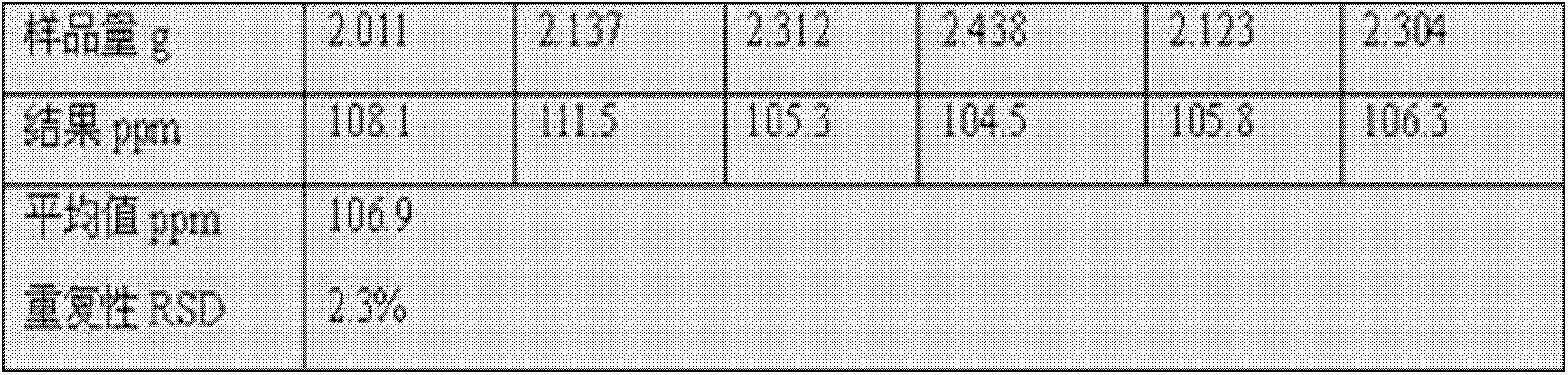Method for detecting free acids in boracic lithium salt and electrolyte of boracic lithium salt
A detection method and free acid technology, applied in the direction of electrochemical variables of materials, etc., can solve the problem of inability to realize the detection of free acid of boron-containing lithium salt and its electrolyte, and achieve the effect of low cost and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Detection of free acid content in lithium difluorooxalate borate.
[0029] A. Configure 0.01mol / L triethylamine-propylene carbonate standard solution:
[0030] Accurately weigh 1.01g (accurate to 0.0001g) of triethylamine, add propylene carbonate solvent to constant volume into a 1000ml volumetric flask, and calibrate its true concentration with benzoic acid reference substance.
[0031] B. Detection of free acid in lithium difluorooxalate borate:
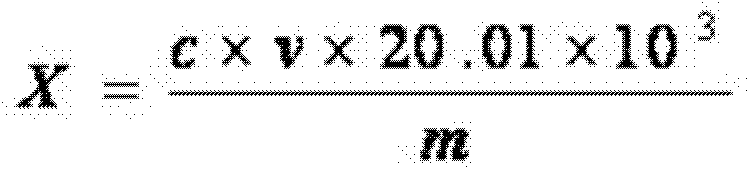
[0032] Accurately weigh 2g of lithium difluorooxalate borate sample (accurate to 0.001g), dissolve in 50ml of high-purity propylene carbonate solvent, stir in a titration cup, and use triethylamine-propylene carbonate with a concentration of 0.01mol / L For the ester standard titration solution, use a Metrohm automatic potentiometric titrator with a PH glass composite electrode, start the automatic titration of the instrument, stop the titration after the test is completed, and calculate the result according to the...
Embodiment 2
[0046] Example 2: Detection of free acid content in lithium bisoxalate borate.
[0047] In this example, the method of Example 1 is adopted, and lithium difluorooxalate borate is replaced with lithium bisoxalate borate, and other steps are identical.
[0048] The examination result of free acid in bisoxalate lithium borate is as shown in table 3:
[0049] Table 3. Content of free acid in lithium bisoxalate borate
[0050]
[0051] The recovery rate test result is as shown in table 4:
[0052] Table 4. Recovery test results
[0053]
Embodiment 3
[0054] Example 3: Detection of free acid content in lithium tetrafluoroborate.
[0055] In this example, the method of Example 1 is adopted, and the lithium difluorooxalate borate is replaced with lithium tetrafluoroborate, and the other steps are exactly the same.
[0056] The examination result of free acid in lithium tetrafluoroborate is as shown in table 5:
[0057] Table 5. Content of free acid in lithium tetrafluoroborate
[0058]
[0059] The recovery rate test result is as shown in table 6:
[0060] Table 6. Recovery test results
[0061]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com