Application of pyrazolopyrimidine compound in preparation of transcription inhibitor medicine
A technology of pyrazolo and inhibitors, applied in the field of pyrazolopyrimidine compounds in the preparation of transcription inhibitor drugs, which can solve the toxic and side effects of human body, the curative effect and prognosis of tumor treatment are not very ideal, and do not have tumor specificity and other problems, to achieve the effect of definite curative effect and good clinical application prospect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
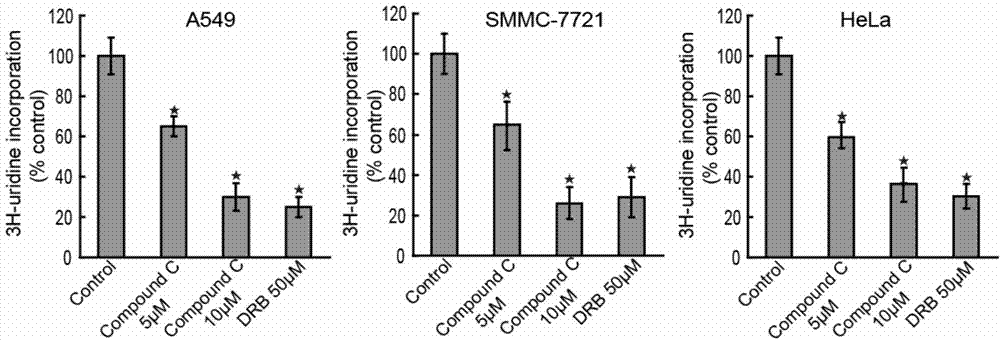
[0030] Example 1 Experiment of Compound C inhibiting transcription
[0031] 1. Experimental method
[0032] (1) Commercially available tumor cells A549, SMMC-7721 and HeLa were taken and cultured;
[0033] The specific culture method is as follows: culture cells in DMEM medium with 10% fetal bovine serum, place in CO 2 in an incubator (5% CO 2 , 95% air, 37°C), change the liquid according to the situation;
[0034] (2) The above cells were treated with 0, 5, and 10 μM / L doses of Compound C, and 3H-uridine (3H-uridine) was added to the medium for 24 hours, and the transcription inhibitor DRB (5 ,6-dichloro-1-β-D-ribofuranosylbenzimidazole) as a positive control;
[0035] (3) Use a liquid scintillation instrument to analyze the amount of 3H-uridine in the cells, and analyze the transcription level.
[0036] 2. Experimental results
[0037] Cells need to use uridine for transcription. Therefore, when 3H-uridine is added to the medium, the higher the cell transcription level...
Embodiment 2
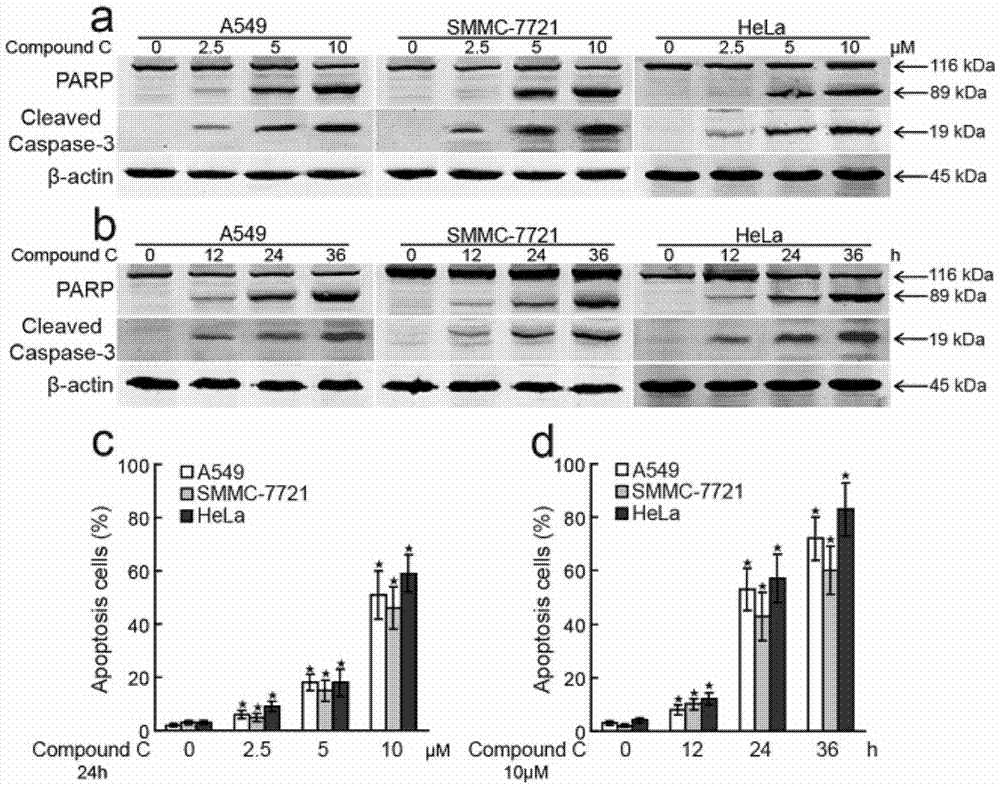
[0040] Example 2 The Experiment of Compound C Promoting Tumor Cell Apoptosis
[0041] 1. Experimental method
[0042] 1.1 Effects of different concentrations of Compound C on apoptotic proteins (PARP, cleaved Caspase-3)
[0043] (1) Commercially available tumor cells A549, SMMC-7721 and HeLa were taken and cultured;
[0044] The specific culture method is as follows: culture cells in DMEM medium with 10% fetal bovine serum, place in CO 2 in an incubator (5% CO 2 , 95% air, 37°C), change the liquid according to the situation;
[0045] (2) Treat the above cells with 0, 2.5, 5, and 10 μm / L doses of Compound C respectively for 24 hours, and collect the cells;
[0046] (3) Cells were taken, lysed, and the obtained lysate was quantified by BCA method, and then denatured by SDS;
[0047] (4) Analyze the above protein samples by SDS-PAGE and Western blot to determine the effect of Compound C on the two apoptotic proteins.
[0048] 1.2 Effects of Compound C treatment for differen...
Embodiment 3
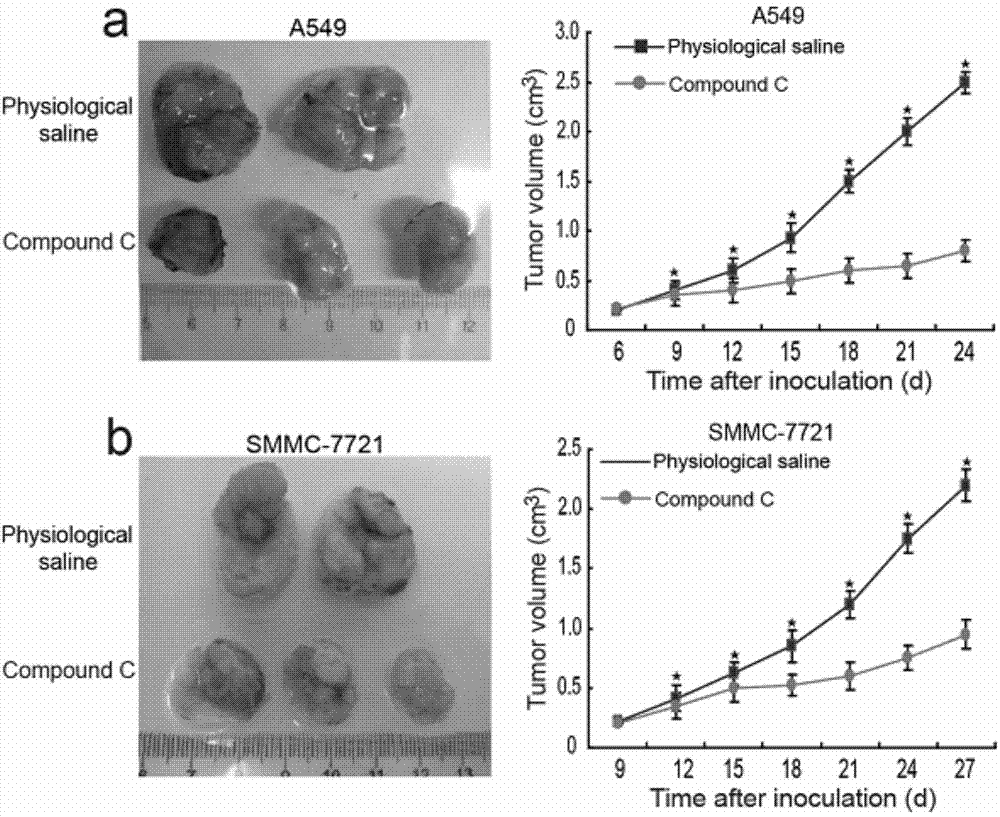
[0069] Example 3 Experiment of Compound C Inhibiting Tumor Formation of Tumor Cells in Vivo
[0070] 1. Experimental animals
[0071] Source and strain: BALB / c-nu nude mice were purchased from the Experimental Animal Center of Second Military Medical University.
[0072] Age, weight: 6 weeks old, about 20g.
[0073] Gender: Male.
[0074] Number of animals: 6 per group.
[0075] 2. Experimental method
[0076] (1) Take commercially available tumor cells A549 and SMMC-7721 and culture them;
[0077] The specific culture method is as follows: culture cells in DMEM medium with 10% fetal bovine serum, place in CO 2 in an incubator (5% CO 2 , 95% air, 37°C) culture;
[0078] (2) Feeding 6-week-old nude mice;
[0079] (3) Take A549 and SMMC-7721 cells and inoculate them subcutaneously in nude mice (cell injection volume is 1×10 7 / Only);
[0080] (4) After 7 days of subcutaneous injection, the experimental group was injected with Compound C intratumorally at a dose of 15 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com