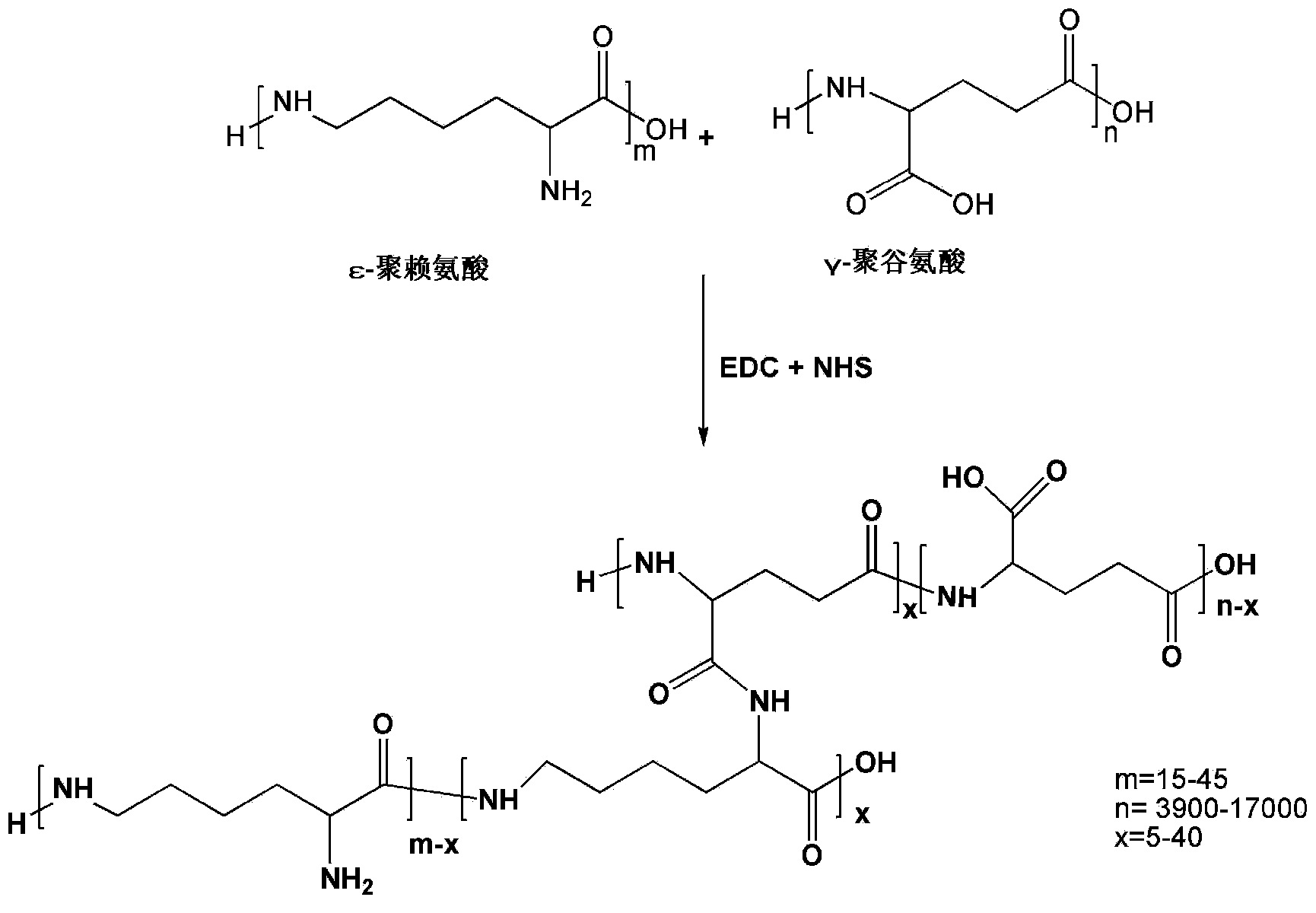
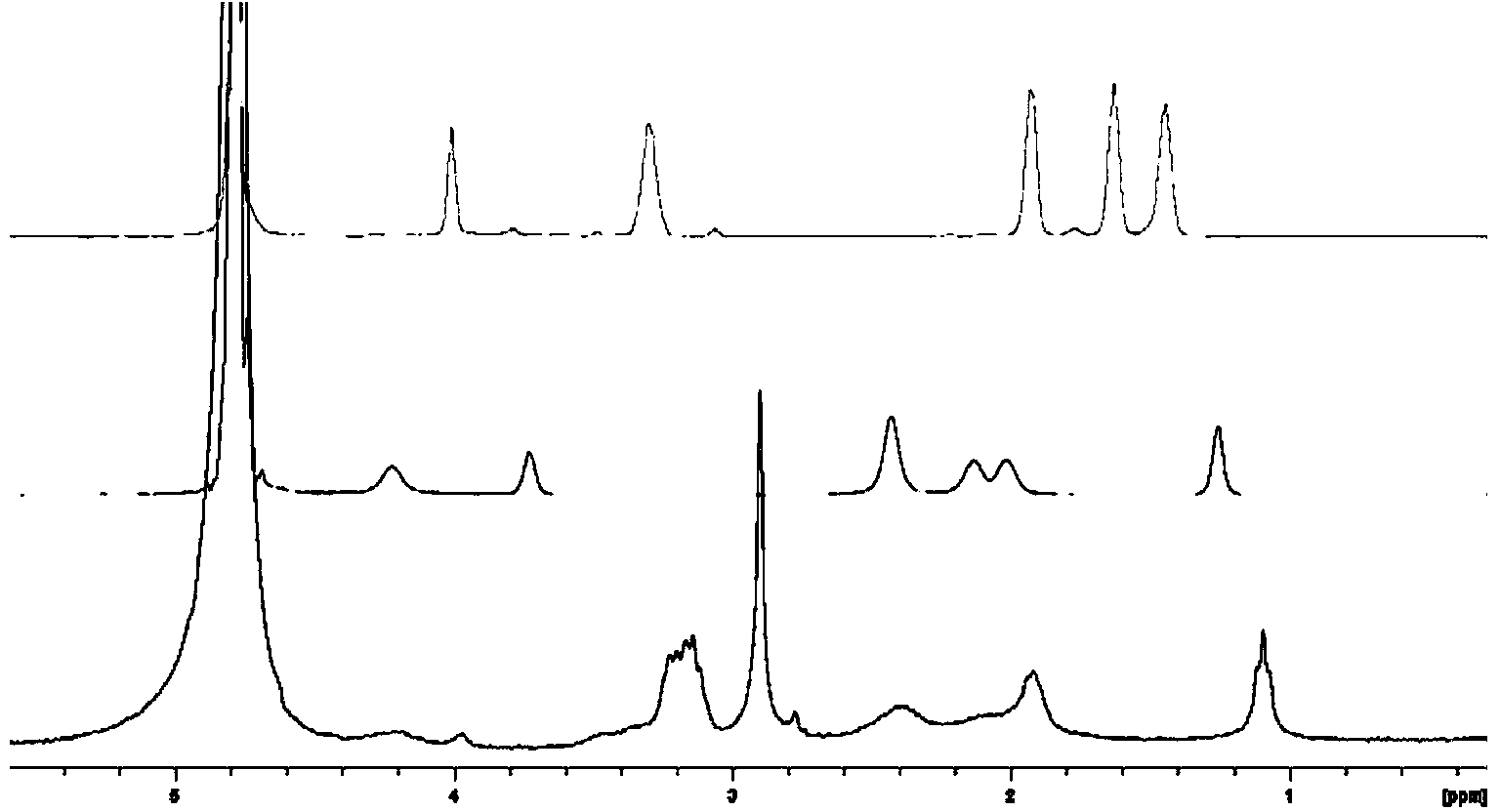
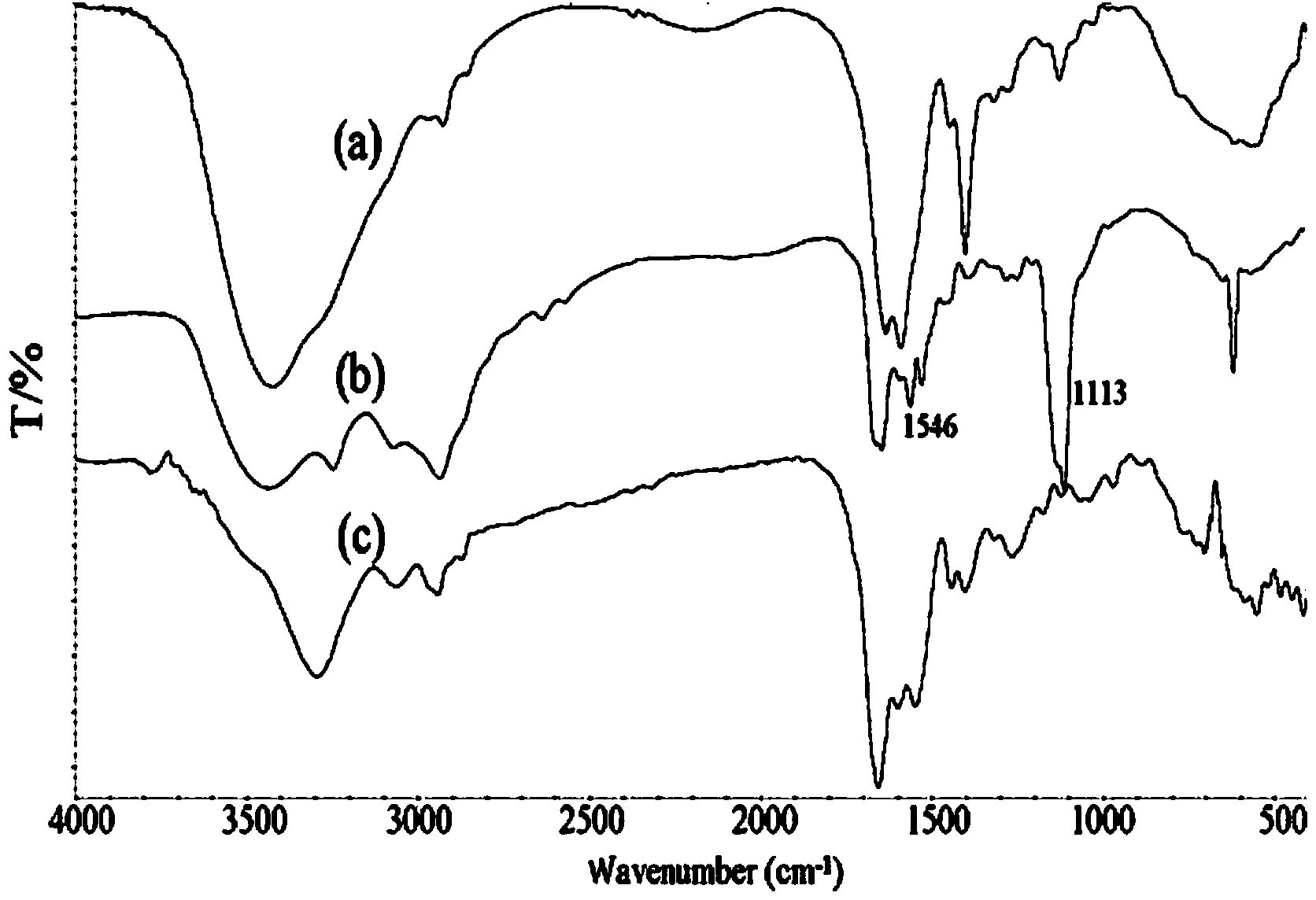
Hydrogel based on gamma-polyglutamic acid and epsilon-polylysine cross-linked polymer and preparation method thereof
A technology of cross-linked polymers and polylysine, applied in bandages, absorbent pads, medical science, etc., can solve problems such as poor biocompatibility, poor biocompatibility of cross-linking agents, and limited application in the medical field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061]Dissolve 4.0 g of γ-polyglutamic acid (1 million to 1.2 million Daltons, containing 0.031 mol carboxyl group) in 50 ml of 0.1 mol / L MES buffer (pH 5.0) at room temperature, and stir until a clear solution is formed. Dissolve 1.78g ε-polylysine (3000-4500 Daltons, containing 0.014mol amino group) in 50ml 0.1mol / L MES buffer (pH5.0) at room temperature, drop ε-polylysine solution drop by drop Add it to the polyglutamic acid solution and stir to mix the solution evenly. Add 4.17g (0.0217mol) of EDC and 2.50g (0.0217mol) of NHS to the mixture of γ-polyglutamic acid and ε-polylysine to control the carboxyl group contained in γ-polyglutamic acid: ε- The molar ratio of amino groups contained in polylysine: EDC: NHS is 1: 0.45: 0.7: 0.7, reacted in ice bath for 30 minutes, and then reacted at room temperature for 2 hours to form a hydrogel. The formed hydrogel was placed in a dialysis bag, dialyzed in deionized water to swelling equilibrium, and then freeze-dried or vacuum-drie...
Embodiment 2
[0063] Dissolve 5.0 g of γ-polyglutamic acid (1 million to 1.2 million Daltons, containing 0.039 mol carboxyl group) in 50 mL of 0.1 mol / L MES buffer (pH 5.0) at room temperature, and stir until a clear solution is formed. Dissolve 1.99g ε-polylysine (3000-4500 Daltons, containing 0.0156mol amino group) in 50mL0.1mol / L MES buffer (pH 5.0) at room temperature, drop ε-polylysine solution drop by drop Add it to the polyglutamic acid solution and stir to mix the solution evenly. Add 4.49g (0.023mol) EDC and 5.08g (0.023mol) sulfo-NHS to the above-mentioned mixture of γ-polyglutamic acid and ε-polylysine to control the carboxyl groups contained in γ-polyglutamic acid: The molar ratio of amino groups contained in ε-polylysine: EDC: Sulfo-NHS is 1: 0.4: 0.6: 0.6, reacted in ice bath for 30 min, and then reacted at room temperature for 2 h to form a hydrogel. The formed hydrogel was placed in a dialysis bag, dialyzed in deionized water to swelling equilibrium, and then freeze-dried o...
Embodiment 3
[0065] Dissolve 6.0 g of γ-polyglutamic acid (1 million to 1.2 million Daltons, containing 0.047 mol of carboxyl groups) in 50 mL of 0.1 mol / L MES buffer (pH 5.0) at room temperature, and stir until a clear solution is formed. Dissolve 3.0g ε-polylysine (3000-4500 Daltons, containing 0.0235mol amino group) in 50mL0.1mol / L MES buffer (pH 5.0) at room temperature, drop the ε-polylysine solution drop by drop Add it to the polyglutamic acid solution and stir to mix the solution evenly. Add 7.95g (0.0188mol) of CMC to the mixture of γ-polyglutamic acid and ε-polylysine to control the carboxyl group contained in γ-polyglutamic acid: the amino group contained in ε-polylysine : The molar ratio of CMC was 1:0.5:0.4, reacted in ice bath for 30min, then reacted at room temperature for 2h to form a hydrogel. The formed hydrogel was placed in a dialysis bag, dialyzed in deionized water to swelling equilibrium, and then freeze-dried or vacuum-dried to obtain a spongy dressing. The expansio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com