Preparation method for nitrogen doped graphene-niobium pentoxide intercalation composite catalyst with high oxygen reduction performance
A niobium pentoxide and intercalation composite technology, which is applied in physical/chemical process catalysts, chemical instruments and methods, chemical/physical processes, etc., can solve the problems of limiting nitrogen-doped graphene and nanoparticles, and achieve the best catalytic effect. , good stability, high methanol tolerance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
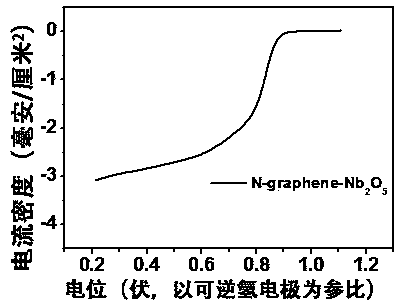
Image
Examples
Embodiment 1
[0032] Niobium pentoxide and potassium carbonate were mixed in a molar ratio of 3:1.1, the resulting mixture was heated to 900°C and maintained for 1 hour, and continued to be heated to 1200°C and maintained for 5 hours. The resulting product was washed with hot water and dried at 200°C for 24 hours. The obtained product was placed in 40 wt% nitric acid solution and stirred for 2 days; repeated 3 times. The resulting product was filtered and washed with distilled water, followed by drying at 50°C for 24 hours. The obtained product was mixed with 4-vinylpyridine at a mass ratio of 1:1, dispersed in water and stirred at room temperature for 3 days. The resulting product was filtered, washed with distilled water, and dried under vacuum at room temperature for 24 hours. The obtained product was heated to 800° C. under an argon atmosphere and maintained for 6 hours, and the target catalyst material could be obtained after cooling.
[0033] Embodiment 1 successfully makes the typ...
Embodiment 2
[0035] Niobium pentoxide and potassium carbonate were mixed in a molar ratio of 3:1.1, the resulting mixture was heated to 900°C and maintained for 1 hour, and continued to be heated to 1200°C and maintained for 5 hours. The obtained product was washed with hot water and dried at 300°C for 24 hours. The obtained product was placed in 40 wt% nitric acid solution and stirred for 2 days; repeated 3 times. The resulting product was filtered and washed with distilled water, followed by drying at 50°C for 24 hours. The obtained product was mixed with 4-vinylpyridine at a mass ratio of 1:1, dispersed in water and stirred at room temperature for 3 days. The resulting product was filtered, washed with distilled water, and dried under vacuum at room temperature for 24 hours. The obtained product was heated to 800° C. under an argon atmosphere and maintained for 6 hours, and the target catalyst material could be obtained after cooling.
Embodiment 3
[0037] Niobium pentoxide and potassium carbonate were mixed in a molar ratio of 3:1.1, the resulting mixture was heated to 900°C and maintained for 1 hour, and continued to be heated to 1200°C and maintained for 5 hours. The resulting product was washed with hot water and dried at 200°C for 24 hours. The obtained product was placed in 40 wt% nitric acid solution and stirred for 3 days; repeated 3 times. The resulting product was filtered and washed with distilled water, followed by drying at 50°C for 24 hours. The obtained product was mixed with 4-vinylpyridine at a mass ratio of 1:1, dispersed in water and stirred at room temperature for 3 days. The resulting product was filtered, washed with distilled water, and dried under vacuum at room temperature for 24 hours. The obtained product was heated to 800° C. under an argon atmosphere and maintained for 6 hours, and the target catalyst material could be obtained after cooling.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com