Preparation method of FeSe nano powder
A nano-powder and solution technology, applied in nanotechnology, nanotechnology, nanotechnology for materials and surface science, etc., can solve the problems of high cost and difficult to promote, achieve low environmental pollution, wide promotion potential, technology short process effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The preparation method of the present embodiment FeSe nanopowder comprises the following steps:
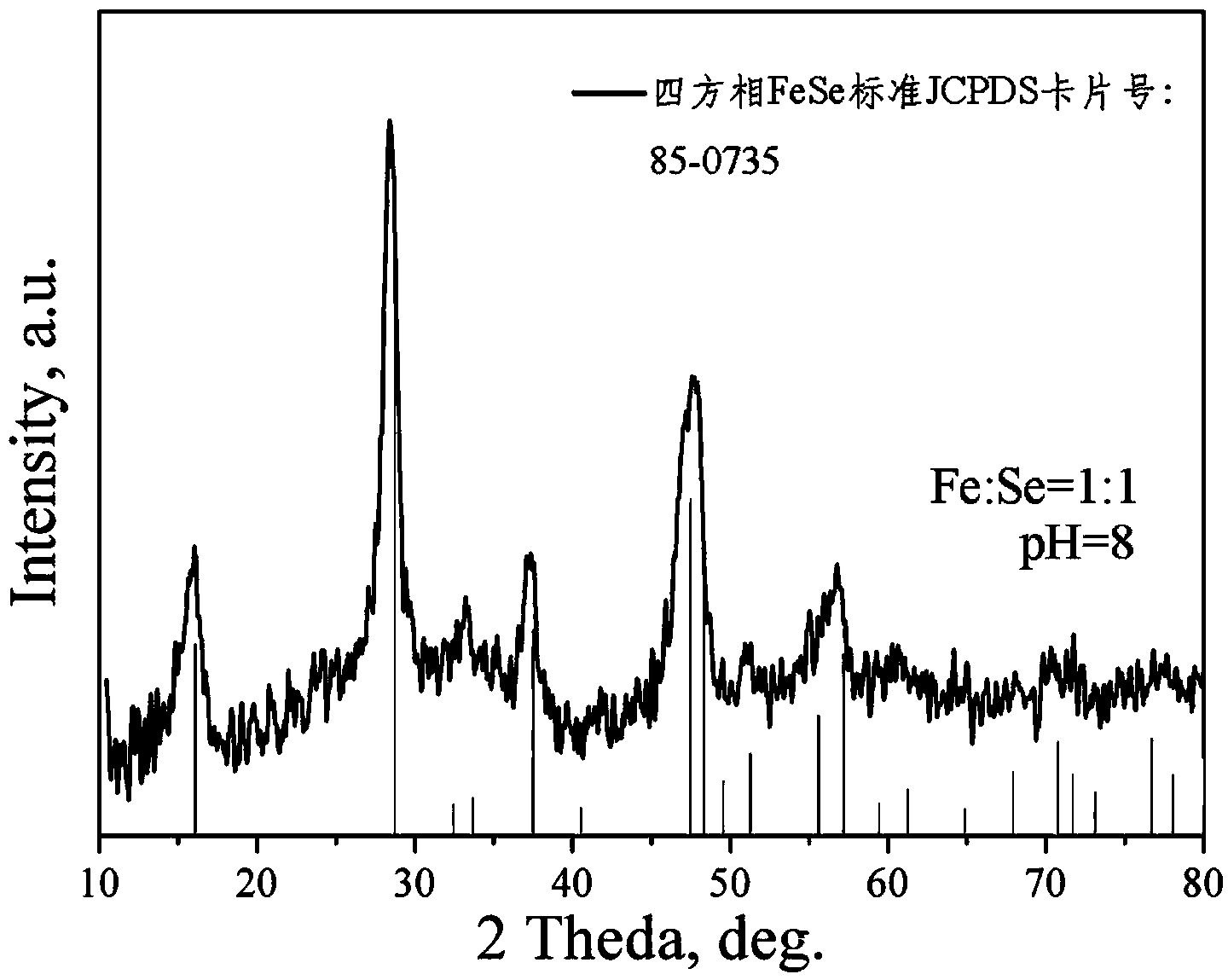
[0040] Step 1. Dissolve 80g NaOH in deionized water to prepare a NaOH solution with a pH value of 8, and then prepare according to Se:OH - =1:2 molar ratio, add 79g of Se powder into the NaOH solution, stir until the Se powder is completely dissolved, and obtain a clear and uniform solution A;
[0041] Step 2. According to NaBH 4 : Se=2:1 molar ratio, 76g NaBH 4 Powder slowly added to solution A in step 1, NaBH 4 The feeding rate of powder is 1.0g / min, stirring until NaBH 4 The powder was completely dissolved to obtain a clear and uniform solution B;
[0042] Step 3, feed inert gas into solution B to remove dissolved oxygen in the solution, then in a glove box full of inert gas, according to Fe 2+ : Se=1:1 molar ratio, 127g FeCl 2 Slowly add in the solution B described in step 2, FeCl 2 The feeding rate is 5g / min, stirring for 30min makes precipitation reaction occur...
Embodiment 2
[0046] The preparation method of the present embodiment FeSe nanopowder comprises the following steps:
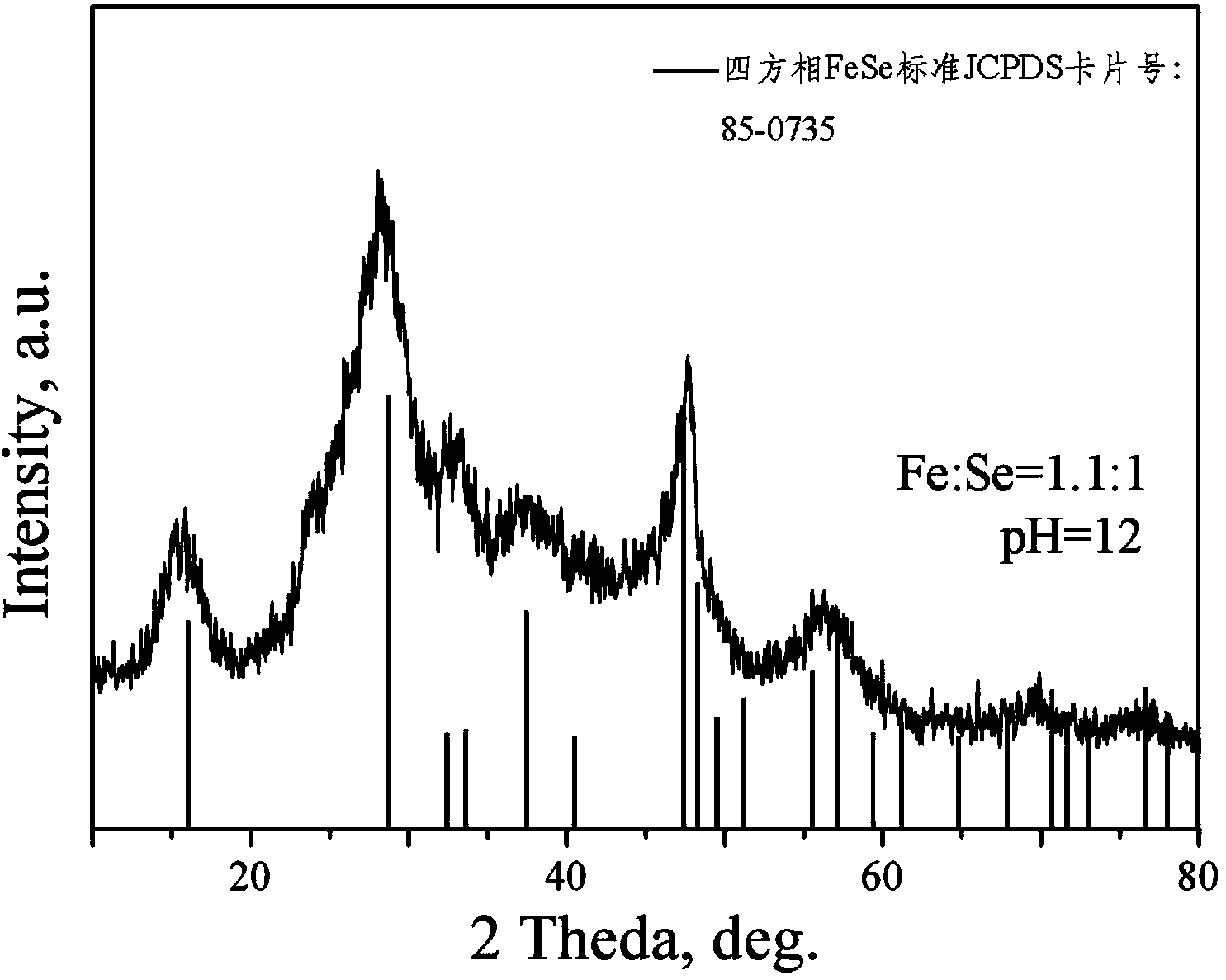
[0047] Step 1. Dissolve 280g KOH in deionized water to prepare a KOH solution with a pH value of 12, and then follow the Se:OH - =1:5 molar ratio, add 79g of Se powder into the KOH solution, stir until the Se powder is completely dissolved, and obtain a clear and uniform solution A;
[0048] Step 2. According to NaBH 4 : Se=3:1 molar ratio, 114g NaBH 4 Powder slowly added to solution A in step 1, NaBH 4 The feeding rate of powder is 1.2g / min, stirring until NaBH 4 The powder was completely dissolved to obtain a clear and uniform solution B;
[0049] Step 3, feed helium into solution B to remove dissolved oxygen in the solution, then in a glove box full of helium, according to Fe 2+ : Se=1.1:1 molar ratio, 167.2g FeSO 4 Slowly add in the solution B described in step 2, FeSO 4 The feeding rate is 6g / min, stirring for 60min makes precipitation reaction occur, and obtain...
Embodiment 3
[0053] The preparation method of the present embodiment FeSe nanopowder comprises the following steps:
[0054] Step 1. Dilute 375g of industrial ammonia water (28wt%) with deionized water to prepare an ammonia solution with a pH value of 9, and then use Se:OH - =1:3 molar ratio, add 79g of Se powder into the ammonia solution, stir until the Se powder is completely dissolved, and obtain a clear and uniform solution A;
[0055] Step 2. According to NaBH 4 : Se=5:1 molar ratio, 190g NaBH 4 Powder slowly added to solution A in step 1, NaBH 4 The feeding rate of powder is 0.8g / min, stirring until NaBH 4 The powder was completely dissolved to obtain a clear and uniform solution B;
[0056] Step 3, feed argon into solution B to remove dissolved oxygen in the solution, then in a glove box full of argon, according to Fe 2+ : Se=1.2:1 molar ratio, 108g Fe(OH) 2 Slowly add in the solution B described in step 2, Fe(OH) 2 The feeding rate is 3g / min, stirring for 45min makes precipi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com