Method for preparing amorphous calcium carbonate nanospheres
A technology of amorphous calcium carbonate and nanospheres, applied in the directions of calcium carbonate/strontium/barium, nanotechnology, nanotechnology, etc., can solve problems such as poor stability of amorphous calcium carbonate, and achieve good application prospects, convenient operation, and simple process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] At room temperature, 0.925 g of CaCl 2 Dissolved in 250 ml of deionized water to form A solution, 1.060 g of Na 2 CO 3 Dissolve in 100 ml of deionized water to form liquid B; take 0.055 g of adenosine triphosphate disodium salt hydrate and dissolve it in 30 ml of solution A and adjust its pH to 9 with 1 mol / L sodium hydroxide solution, then add 10 ml of B dropwise During this process, magnetic stirring was used to keep the pH value at about 9; the dropwise addition was completed, stirred at room temperature for 1 hour, and then centrifuged, and the separated solid was washed 3 times with deionized water, and then washed with absolute ethanol Wash once, and finally dry in air at 60°C.
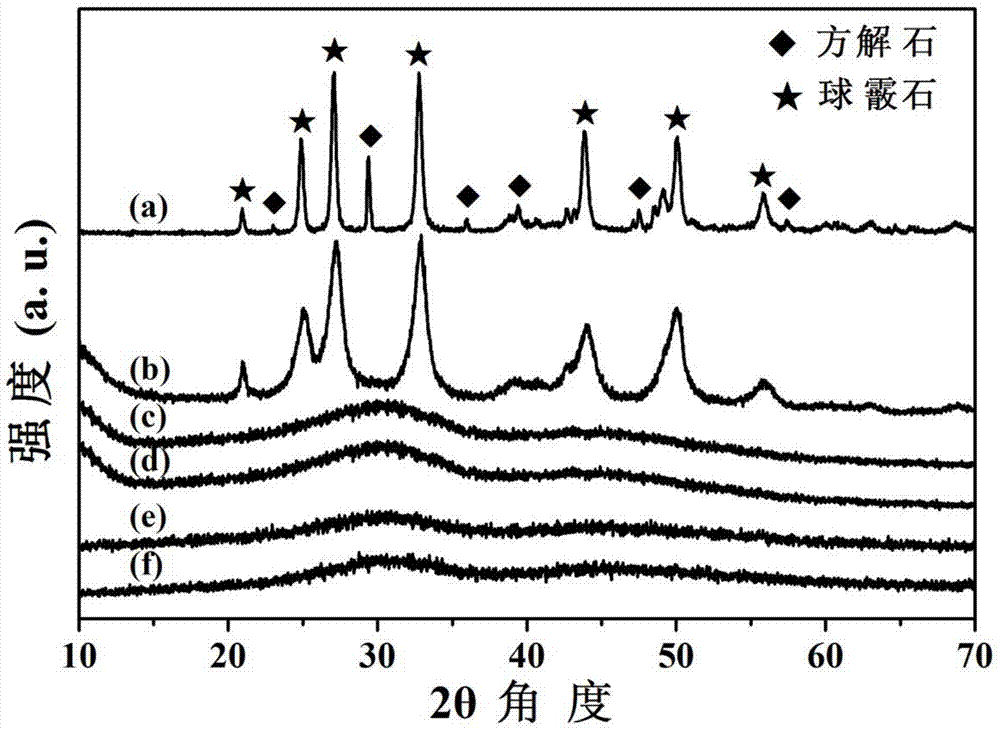
[0045] The X-ray (XRD) diffraction pattern of the obtained sample is as follows figure 1 Shown in the curve c in: the obtained sample is an amorphous phase.
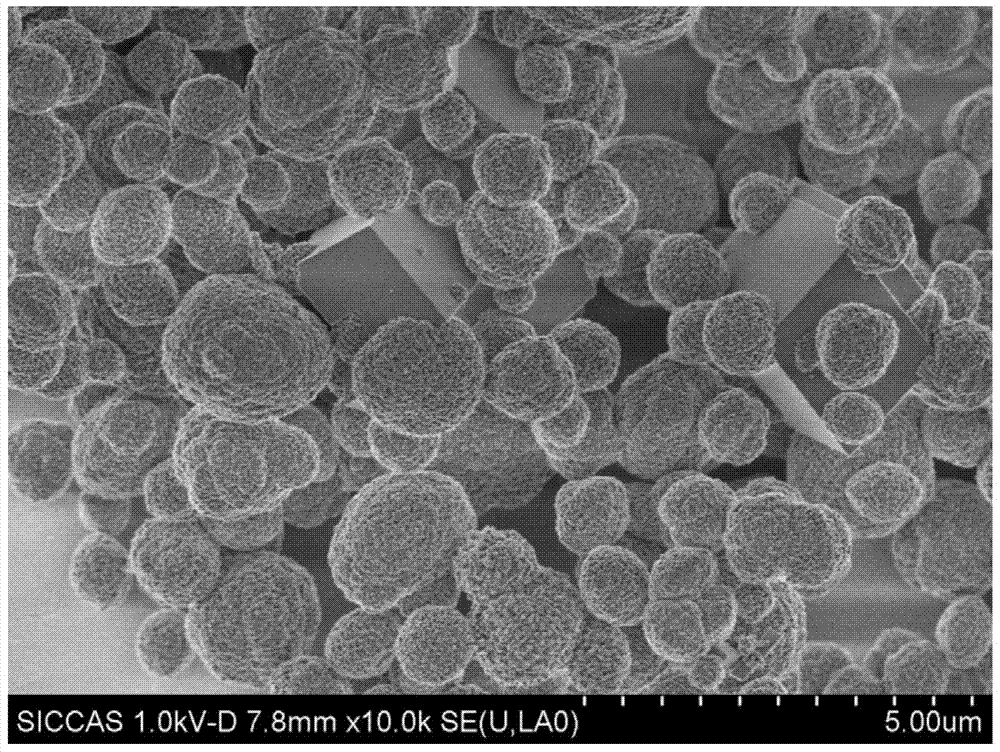
[0046] The SEM photographs of the obtained samples are as Figure 4 Shown: the obtained sample is a calcium carbonate nanosph...
Embodiment 2
[0048] At room temperature, 0.925 g of CaCl 2 Dissolved in 250 ml of deionized water to form A solution, 1.060 g of Na 2 CO 3Dissolve in 100 ml of deionized water to form liquid B; dissolve 0.088 g of adenosine triphosphate disodium salt hydrate in 30 ml of solution A and adjust the pH to 9 with 1 mol / L sodium hydroxide, then add 10 ml of liquid B dropwise In this process, magnetic stirring is used and the pH value is kept at about 9; the dropwise addition is completed, stirred at room temperature for 1 hour, and then centrifuged, and the separated solid is washed 3 times with deionized water, and then washed with absolute ethanol 1 time, and finally dry in air at 60°C.
[0049] The X-ray (XRD) diffraction pattern of the obtained sample is as follows figure 1 Shown in the curve d in: the obtained sample is an amorphous phase.
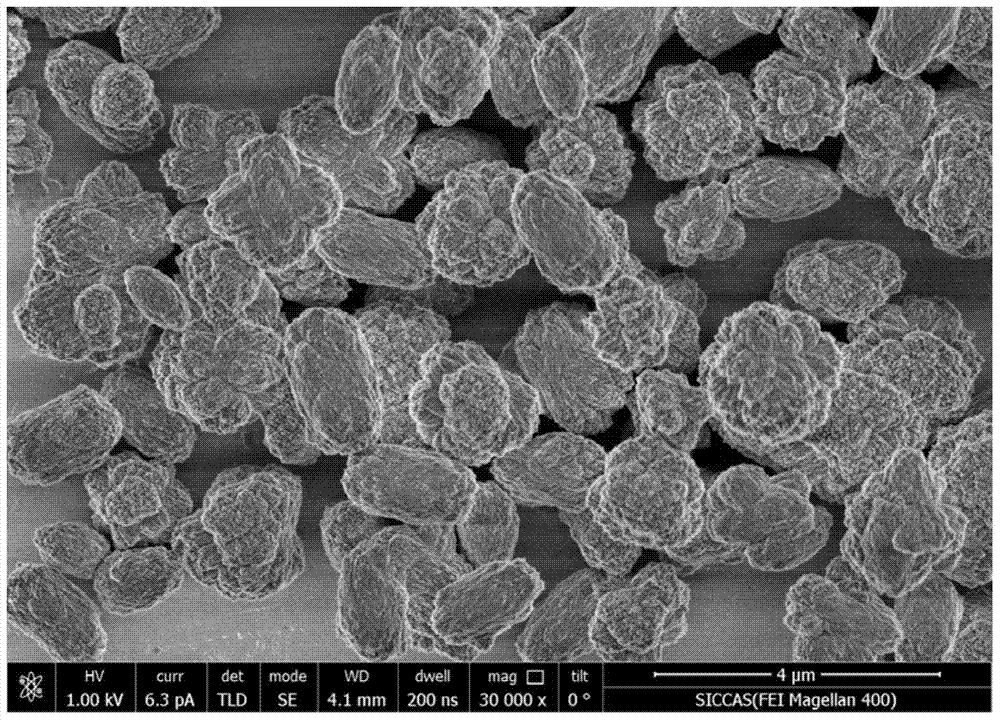
[0050] The SEM photographs of the obtained samples are as Figure 5 Shown: the obtained sample is a calcium carbonate nanosphere with a diameter o...
Embodiment 3
[0052] At room temperature, 0.925 g of CaCl 2 Dissolved in 250 ml of deionized water to form A solution, 1.060 g of Na 2 CO 3 Dissolve in 100 ml of deionized water to form liquid B; take 0.110 g of adenosine triphosphate disodium salt hydrate and dissolve it in 30 ml of solution A and adjust its pH to 9 with 1 mol / L sodium hydroxide, then add 10 ml of liquid B dropwise In this process, magnetic stirring is used and the pH value is kept at about 9; the dropwise addition is completed, stirred at room temperature for 1 hour, and then centrifuged, and the separated solid is washed 3 times with deionized water, and then washed with absolute ethanol 1 time, and finally dry in air at 60°C.
[0053] The X-ray (XRD) diffraction pattern of the obtained sample is as follows figure 1 Shown in the curve e in: the obtained sample is an amorphous phase.
[0054] The SEM photographs of the obtained samples are as Image 6 Shown: the obtained sample is a calcium carbonate nanosphere with ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com