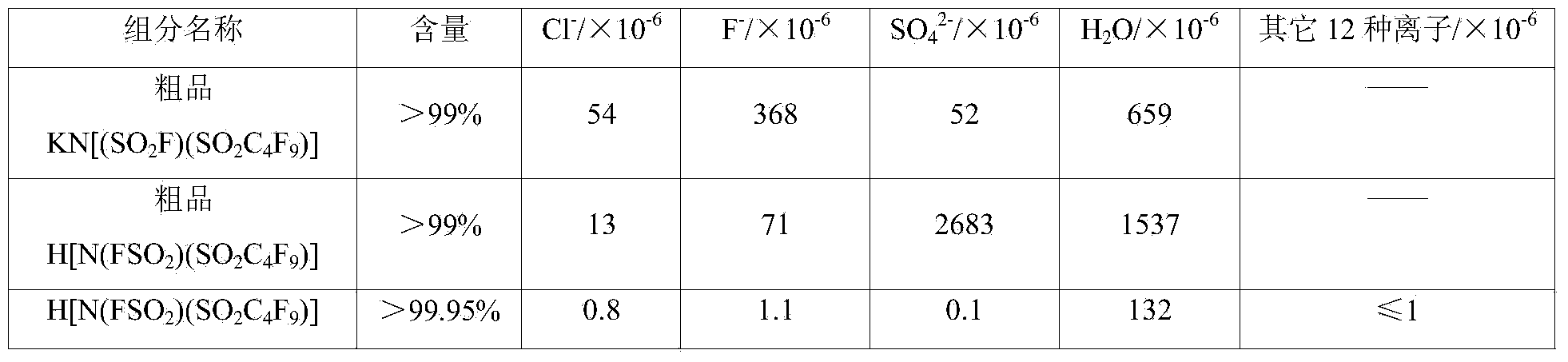Method and device for preparing fluorine sulfimide acid
A technology of fluorosulfonylimide acid and fluorosulfonylimide salt, which is applied in the field of preparation of fluorosulfonylimide acid, can solve problems such as poor safety, insufficient purity of fluorosulfonylimide acid, and inconvenient operation of the method. Achieve the effects of preventing solidification, improving reaction and distillation efficiency, and reducing the content of impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
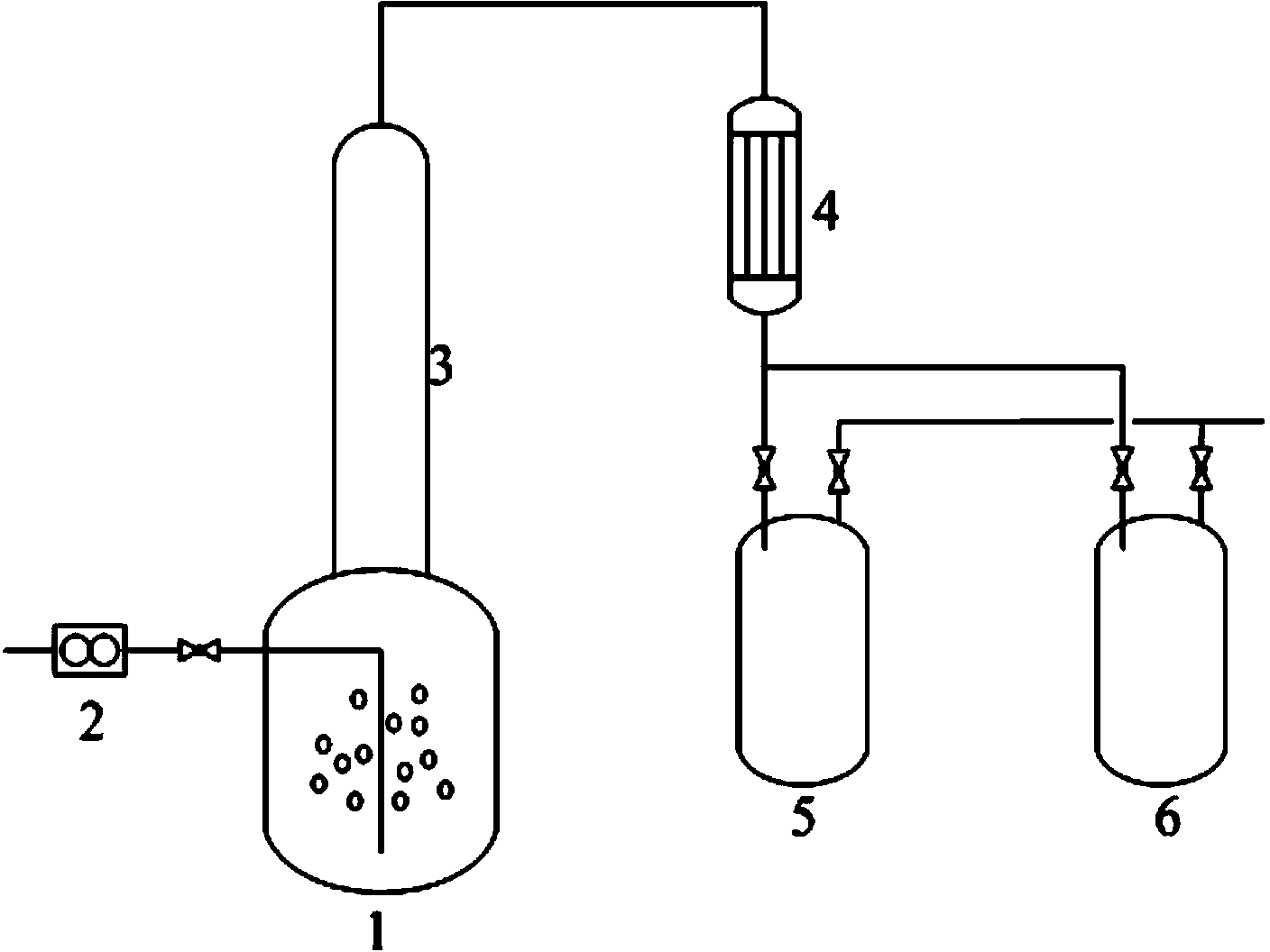
[0045] 11.4kg (0.116kmol) mass concentration is 100% sulfuric acid, 14.8kg (0.0459kmol) crude product KN (SO 2 CF 3 ) 2 And 1kg of silicon dioxide was put into a 20L reaction vessel for reaction. Stirring was kept during the reaction and distillation process, and nitrogen gas was introduced. The reaction temperature was 85-87°C. The reaction was 4h, and the crude product HN(SO 2 CF 3 ) 2 12.5kg, the yield is 96%. The conditions for feeding nitrogen are: the flow rate is 0.5-0.6L / min, and the pressure is 0-0.1MPa. The crude product KN(SO 2 CF 3 ) 2 And the obtained crude product HN(SO 2 CF 3 ) 2 The mass content of the components is shown in Table 3.
[0046] The mass content table of each material component of table 3 embodiment 1
[0047] component name
[0048] The rectification tower used for purification and the internal connecting pipes of the device are made of stainless steel. The volume of rectification kettle 1 is 10L, the diameter of rectificati...
Embodiment 2
[0050] 212.45kg (2.1245kmol) of sulfuric acid with a mass concentration of 98%, 940kg (4.249kmol) of crude product KN(SO 2 F) 2 And 78.33kg of silicon dioxide was put into a 1000L reaction vessel for reaction. Stirring was kept during the reaction and distillation process, and nitrogen gas was introduced. The reaction temperature was 145-150°C. The reaction was 1h, and the crude product HN(SO 2 F) 2 730kg, the yield is 94.0%, and the conditions for feeding nitrogen are: flow rate 3-6L / min, pressure 0-0.5MPa. The crude product KN(SO 2 F) 2 And the obtained crude product HN(SO 2 F) 2 The mass content of the components is shown in Table 4.
[0051] The mass content table of each material component of table 4 embodiment 2
[0052] component name
[0053] The rectification tower used for purification and the internal connecting pipes of the device are made of stainless steel. The volume of rectification kettle 1 is 1000L, the diameter of rectification column 3 is 2...
Embodiment 3
[0055] 6.628 (71.0185mol) kg of sulfuric acid with a mass concentration of 105%, 10kg (14.2037mol) of crude product Ba[N(SO 2 CF 3 ) 2 ] 2 and 0.4kg of silicon dioxide were put into a 10L reaction vessel, kept stirring during the reaction and distillation process, and nitrogen gas was introduced, the reaction temperature was 90-93°C, the reaction was 2h, and the crude product HN(SO 2 CF 3 ) 2 7.82kg, the yield is 97%. The conditions for feeding nitrogen are: flow rate 1.6-2.0L / min, pressure 0.30-0.33MPa. The crude product Ba[N(SO 2 CF 3 ) 2 ] 2 And the obtained crude product HN(SO 2 CF 3 ) 2 The mass content of the components is shown in Table 5.
[0056] The rectification tower used for purification and the internal connecting pipes of the device are made of stainless steel. The volume of rectification kettle 1 is 10L, the diameter of rectification column 3 is 20mm, and the height is 500mm. . In this rectifying kettle 1, add the crude product HN(SO) of 7.8kg 2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com