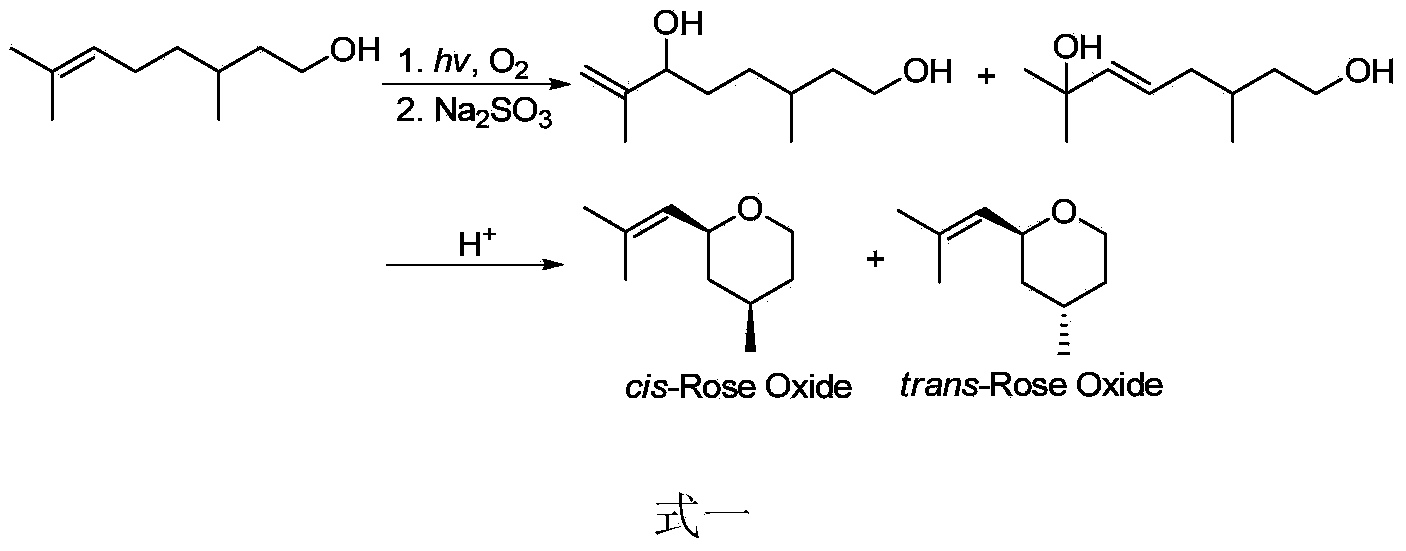
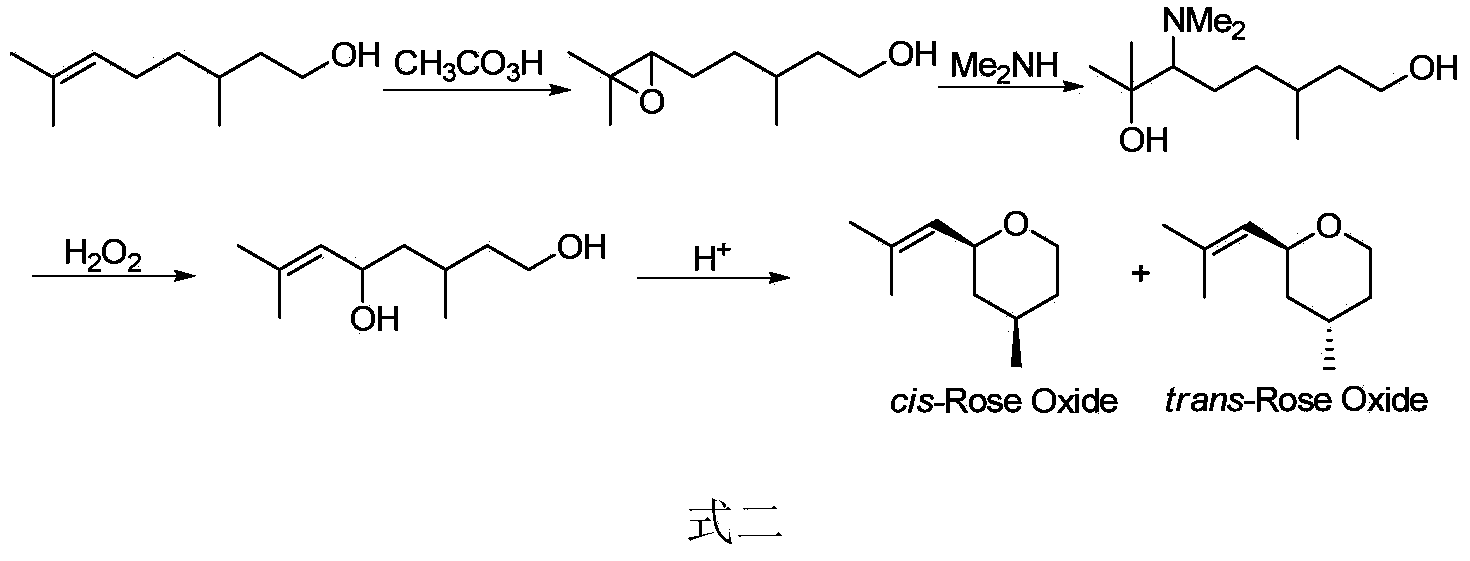
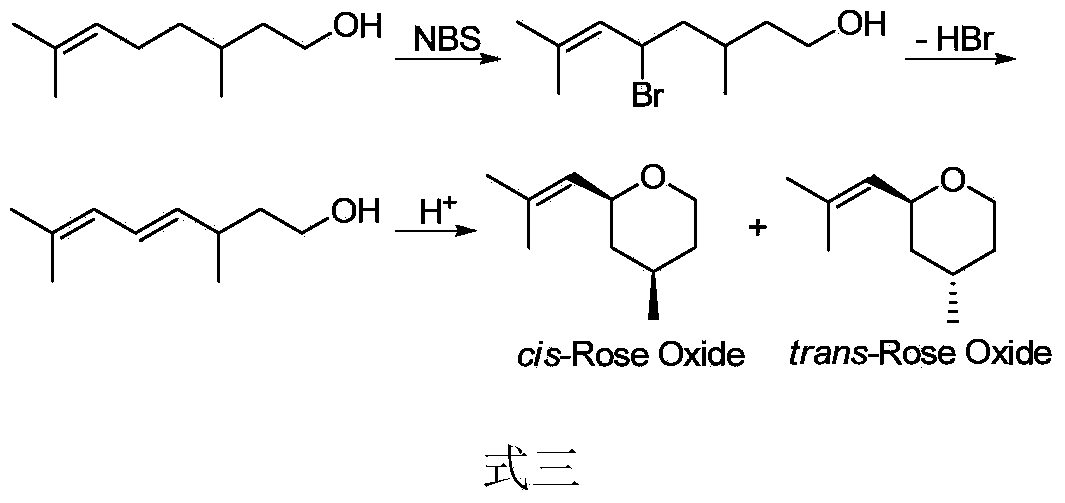
Preparation method of rose ether
A technology of rose ether and compounds, which is applied in the field of preparation of rose ether, can solve the problems of low reaction yield, increased production cost, and low stereoselectivity, and achieve the effects of high reaction yield, low production cost, and convenient processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] The preparation method of pure cis-rose ether
[0071] 1) Preparation of corresponding α,β-unsaturated ketones:
[0072] Citronellol (1.0g, 6.4mol) was dissolved in CH 2 Cl 2 (5mL), m-CPBA (1.1g, 6.4mmol) was slowly added in batches at 0°C, and after 30 minutes, diethyl ether (200mL) was added, and the mixture was washed with saturated Na 2 CO 3 solution and saline wash. Anhydrous Na for organic phase 2 SO 4 After drying, the solvent was evaporated under reduced pressure to obtain an epoxy compound.
[0073] The citronellol used in the reaction can be in R configuration, S configuration or a mixture of R configuration and S configuration, and m-CPBA can be replaced by peracetic acid or peroxybenzoic acid.
[0074] Under the protection of argon, the epoxy compound was dissolved in toluene (4 mL), aluminum isopropoxide (1.3 g, 6.4 mmol) was added, and the temperature was raised to reflux for 8 hours. The system was cooled to room temperature and acidified with 2M ...
Embodiment 2
[0091] Preparation method of cis-rose ether and trans-rose ether mixture
[0092] The specific operations of steps 1) and 2) are the same as the steps 1) and 2) of Example 1, the difference lies in step 3), and the specific operations of step 3) are as follows:
[0093] Allene compound (0.77g, 5.0mmol) was dissolved in 10mL of toluene at room temperature, hydrochloric acid (10mmol) was added slowly, and reacted for 20 hours. Add 5 mL of saturated NaHCO 3 The reaction was terminated with the solution, extracted with ether (100 mL), and the organic phase was washed with brine. Anhydrous Na 2 SO 4 After drying, the solvent was evaporated under reduced pressure, and the mixture of cis-rose ether and trans-rose ether (0.38g, 50% yield) was obtained by column chromatography. The ratio of cis-product to trans-product was 1:1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com