Naphthalimide modified rhodamine B derivative as well as preparation method and application thereof
A technology of naphthalimide and derivatives, which is applied in the field of fluorescent probes, can solve problems such as the influence and inconvenience of metal ion detection, no fluorescent probes have been developed and reported, and the interference of coexisting ions is large, achieving low cost and high yield High, high-sensitivity effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
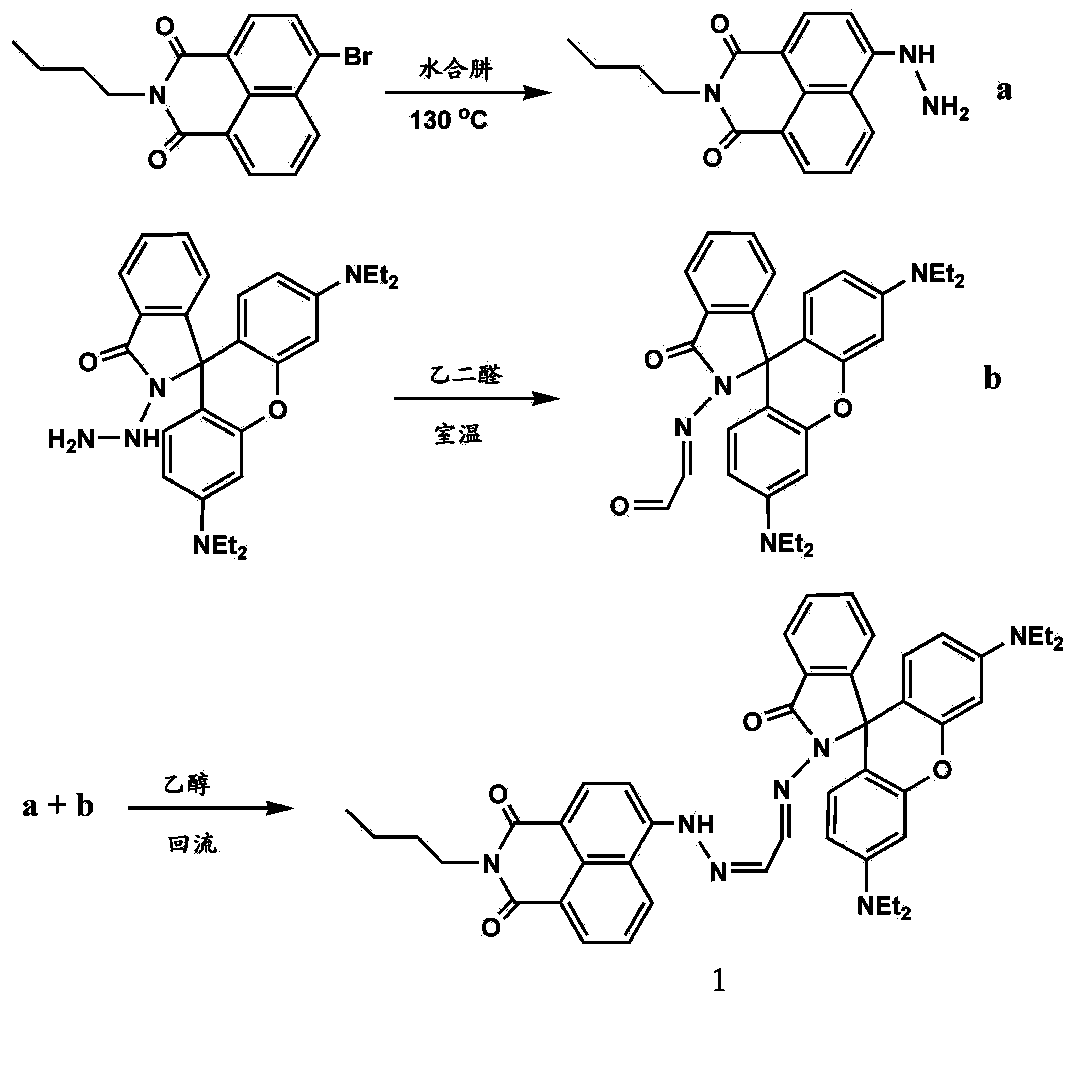
[0020] The rhodamine B derivative modified by naphthalimide is a compound shown in formula 1:
[0021]
[0022] Formula 1.
[0023] Preparation of rhodamine B derivatives modified by naphthalimide:
[0024] React N-butyl-4-bromo-1,8-naphthalimide with excess hydrazine hydrate at 130°C for 6 hours, cool and precipitate the solid, filter it, and wash it with water. The obtained product a is directly used in the next reaction [1];
[0025] The rhodamine B hydrazinolysis product and glyoxal were reacted at room temperature for 2 hours in absolute ethanol at a molar ratio of 1:10, and the solvent was evaporated under reduced pressure. The crude product was separated by chromatographic column with petroleum ether and ethyl acetate as the eluent to obtain the yellow intermediate product b, the yield: 72.5%; wherein petroleum ether and ethyl acetate were mixed at a volume ratio of 5:1;
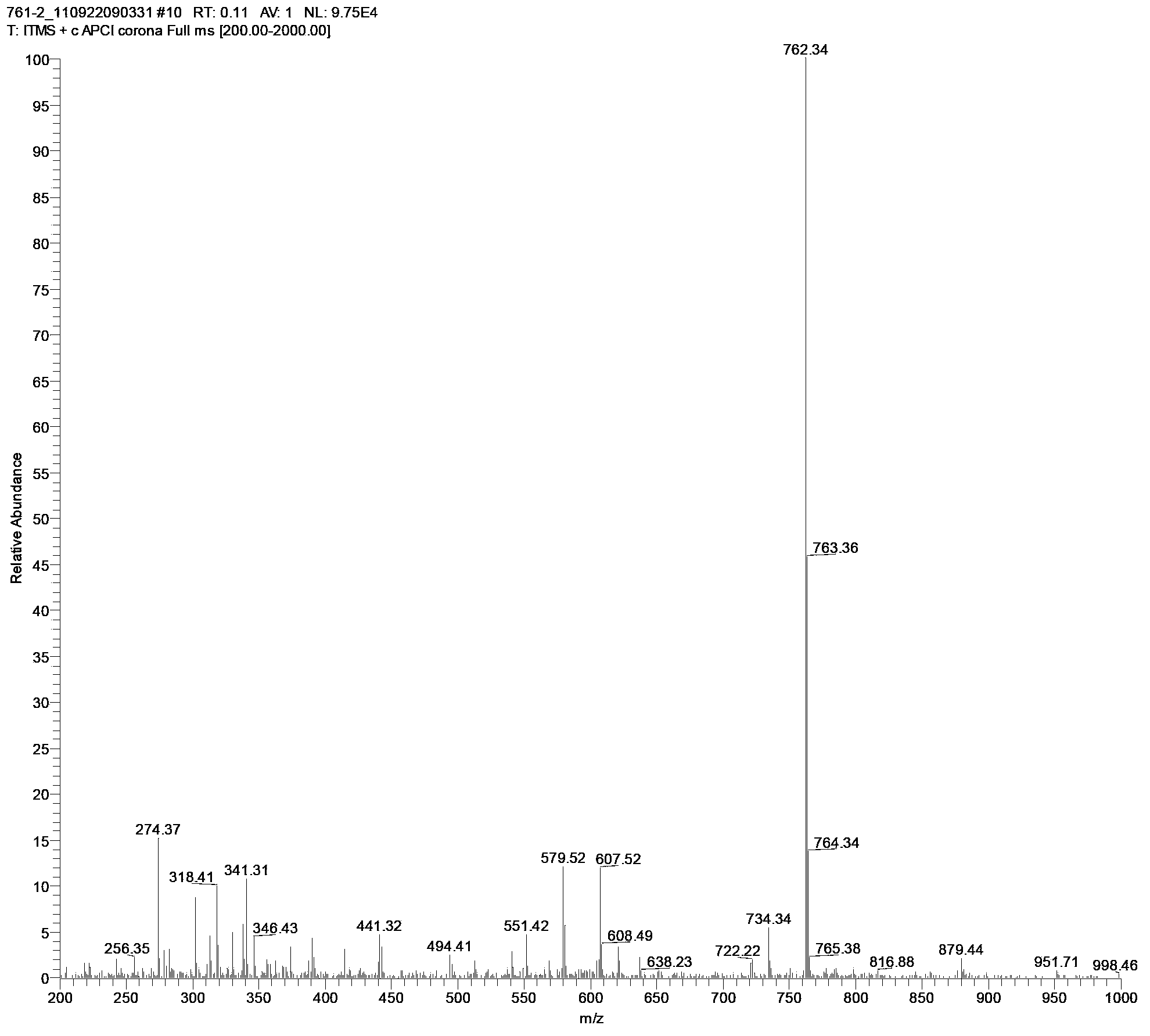
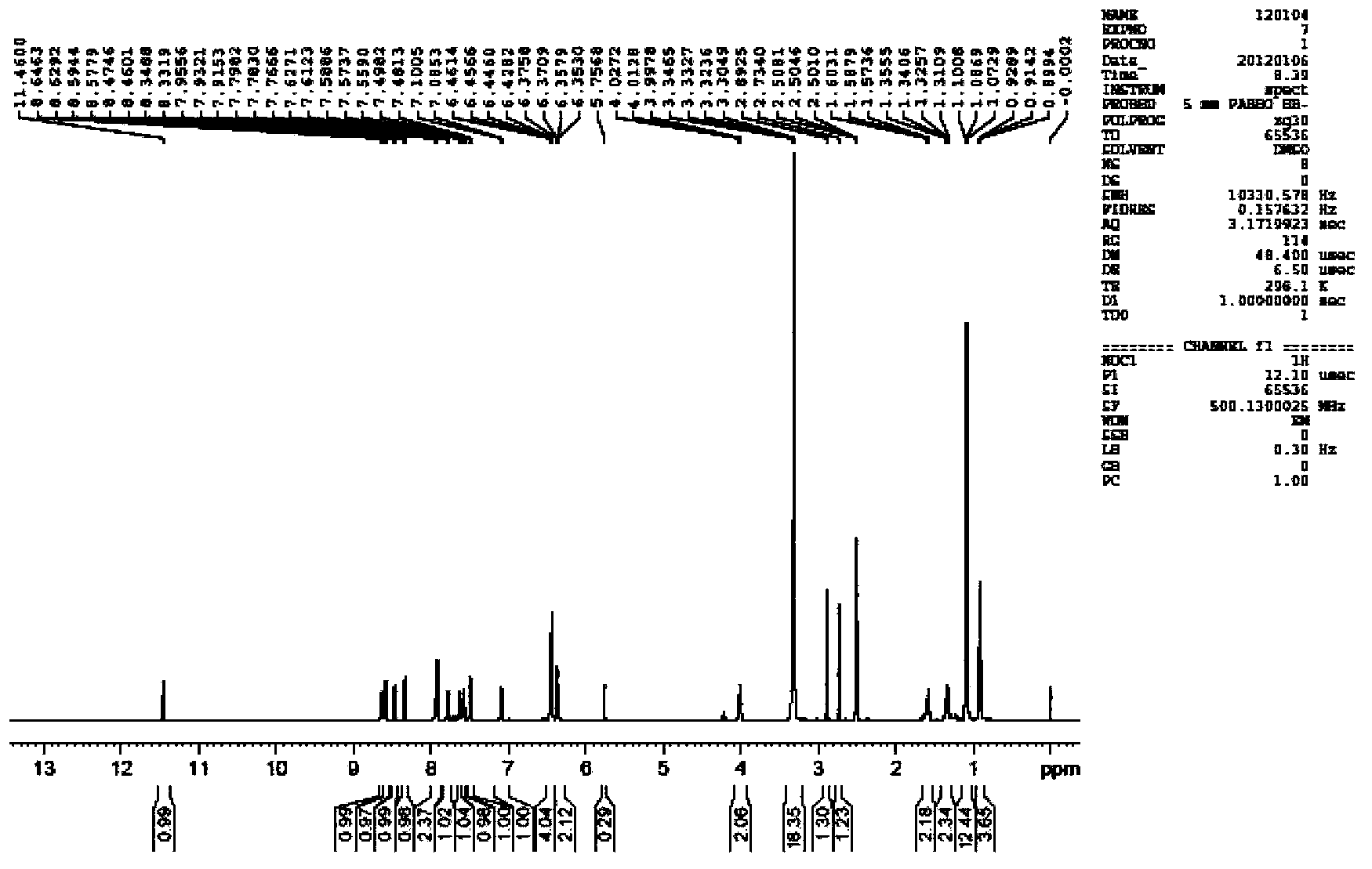
[0026] The above-mentioned intermediate compound a and b were reacted in absolute ethanol at ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com