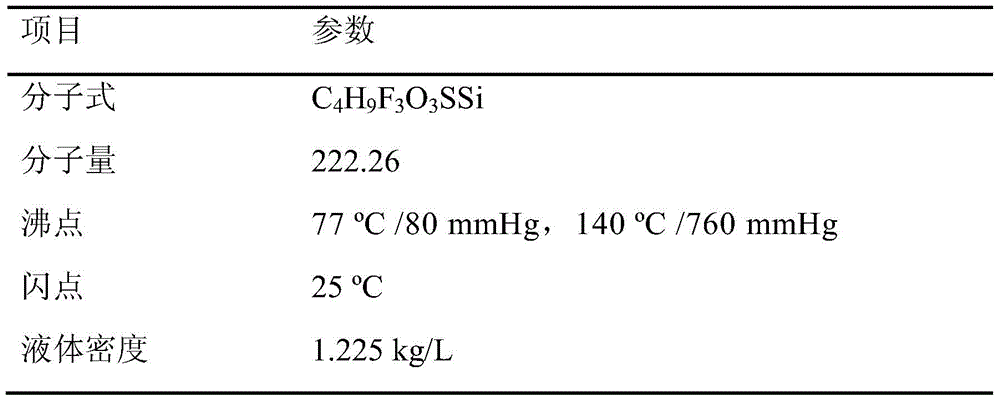
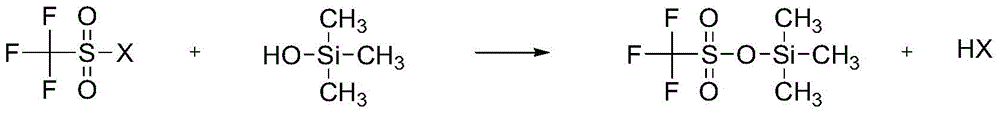
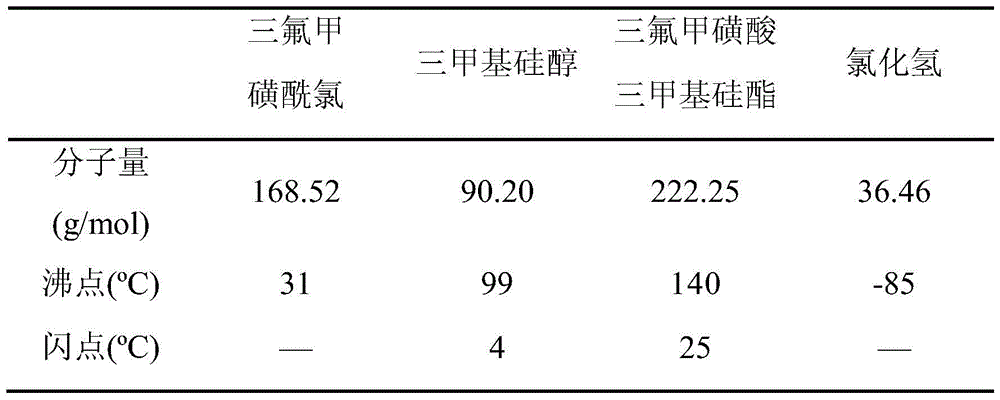
A kind of preparation method of trimethylsilyl trifluoromethanesulfonate
A technology of trimethylsilyl trifluoromethanesulfonate and trimethylsilanol, which is applied in the direction of silicon organic compounds, and can solve the problem of low quality of trimethylsilyl trifluoromethanesulfonate products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~15
[0062] Product detection method in following embodiment 1~15:
[0063] The obtained product was analyzed qualitatively and quantitatively by a Bruker 300 MHz nuclear magnetic resonance spectrometer. The product described below refers to a product whose composition is mainly trimethylsilyl trifluoromethanesulfonate after distillation of the reaction mixture.
[0064] The fluorine spectrum is the in situ spectrum of the pure product, and the hydrogen spectrum uses CDCl 3 as a solvent.
Embodiment 1
[0066] Step 1: In a 1000mL three-neck round bottom flask, add 350g of trifluoromethanesulfonyl chloride, and drop 185mL of trimethylsilanol into trifluoromethanesulfonyl chloride with a constant pressure dropping funnel, and the time for adding trimethylsilanol is 0.5 Hours, the molar ratio of trifluoromethanesulfonyl chloride and trimethylsilanol is 1.00:0.80, the reaction temperature is -50 ° C, stirring, HCl gas escapes, and the generated HCl gas is absorbed with NaOH solution. Stirring was continued, and the reaction time was 10 hours. After the reaction was completed, a reaction mixture was obtained.
[0067] Step 2: The reaction mixture was distilled under reduced pressure. After the distillation pressure was reduced to 0.030 MPa, the temperature of the reaction mixture was raised to 40° C. and stirred for 0.5 hour to remove unreacted trifluoromethanesulfonyl chloride. Then the temperature of the reaction mixture was raised to 70° C., stirred for 0.5 hour, and trimethyls...
Embodiment 2
[0070] Step 1: In a 1000mL three-neck round bottom flask, add 350g of trifluoromethanesulfonyl chloride, drop 243mL of trimethylsilanol into the trifluoromethanesulfonyl chloride with a constant pressure dropping funnel, and add trimethylsilanol for 0.5 Hours, the molar ratio of trifluoromethanesulfonyl chloride and trimethylsilanol is 1.00:1.05, the reaction temperature is 10 ° C, stirring, HCl gas escapes, and the generated HCl gas is absorbed with NaOH solution. Stirring was continued, and the reaction time was 4 hours. After the reaction was completed, a reaction mixture was obtained.
[0071] Step 2: The reaction mixture was distilled under reduced pressure. After the distillation pressure was reduced to 0.015 MPa, the temperature of the reaction mixture was raised to 40° C. and stirred for 0.5 hour to remove unreacted trifluoromethanesulfonyl chloride. The temperature of the reaction mixture was raised to 70° C., stirred for 0.5 hours, and trimethylsilanol was removed. F...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com