Novel tumour serum marker and application thereof
A technology for serum markers and tumors, applied in biological testing, biomaterial analysis, peptide preparation methods, etc., can solve problems such as limiting clinical application value, unclear early detection of tumors, unclear CT antigen autoantibodies, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Example 1, Prokaryotic expression vector construction, expression and purification of POTE fragment antigen
[0065] (1) Cloning of POTE gene cDNA
[0066] HELA cell mRNA was extracted with TRIzol reagent and reverse-transcribed (RT) into cDNA. Using this cDNA as a template, the cDNA fragments of two subtypes 2A and 2C of POTE were amplified by PCR. After determination, their sequences were shown as SEQ ID No.13 (POTE-2A ORF sequence) and SEQ ID No.14 (POTE-2C ORF sequence) shown.
[0067] (2) Construction of prokaryotic expression vectors for POTE polypeptide fragments
[0068] By PCR method, the gene sequence encoding POTE-E protein molecule 1-144aa (or 2-144aa) (see SEQ ID No.10), the gene sequence encoding POTE-E protein molecule 390-524aa region (see SEQ ID No.11) and the gene sequence encoding the 528-641aa region of the POTE-E protein molecule (see SEQ ID No.12) were cloned into the pGEX-6P-3 vector to construct pGEX-GST-POTE_144, pGEX-GST-POTE_390 and pGEX- ...
Embodiment 2
[0071] Example 2, Purification and identification of POTE autoantibodies
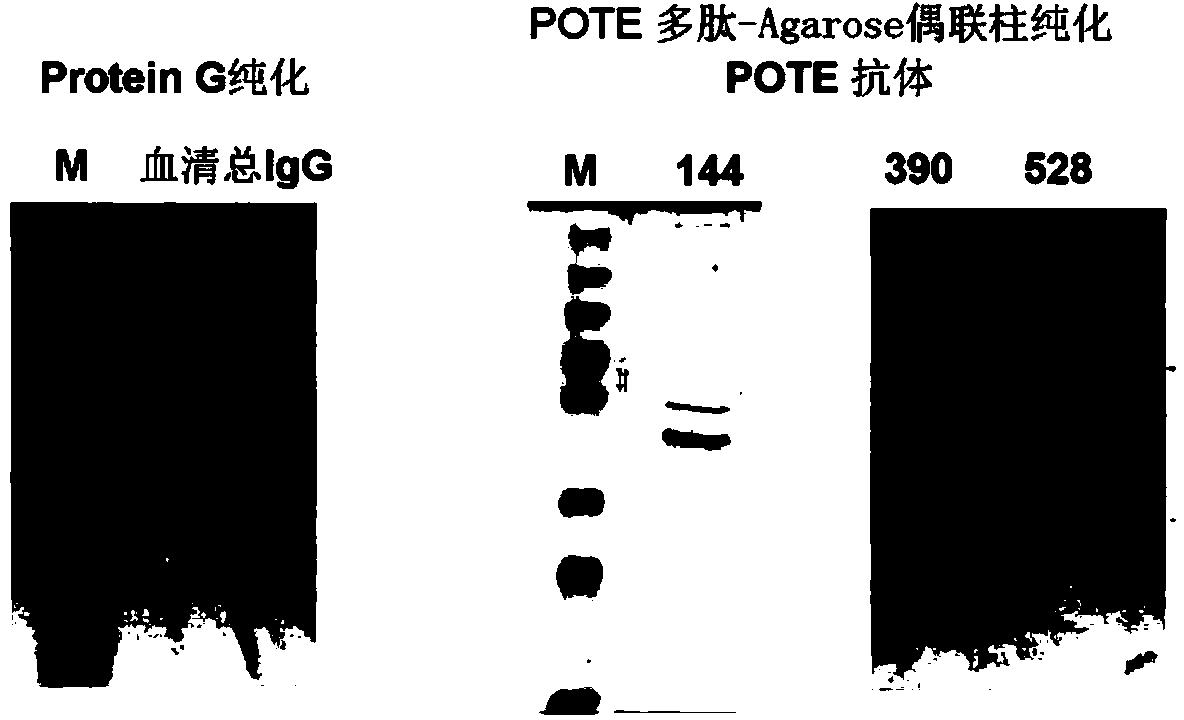
[0072] In this example, in order to prove the existence of POTE autoantibodies, a solid-phase affinity chromatography column for POTE polypeptide fragments was first prepared, and this column was used to enrich and purify tumor patient serum to obtain POTE autoantibodies. The specific operation is as follows:
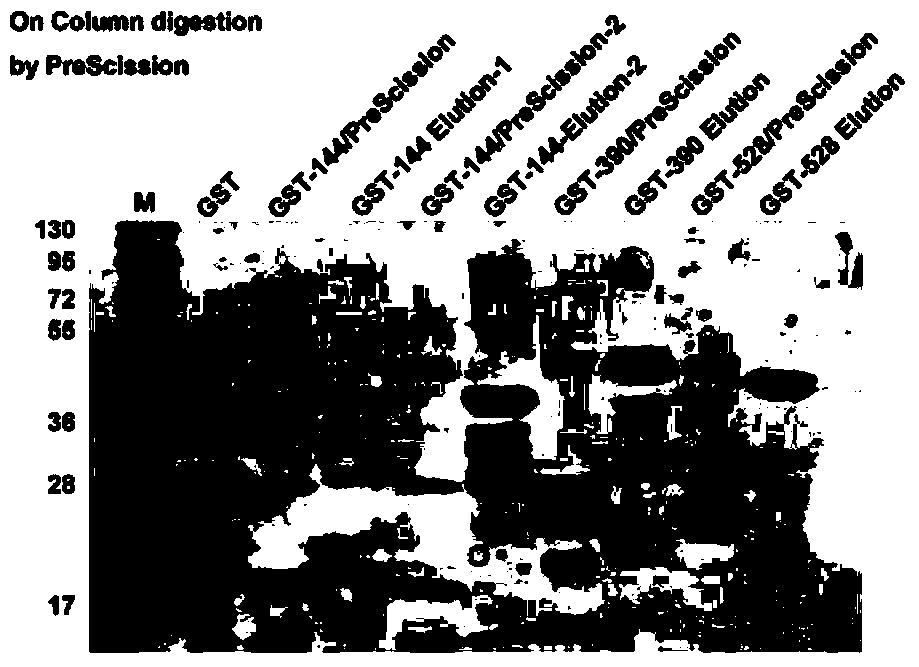
[0073] Preparation of POTE polypeptide fragment solid-phase affinity chromatography column: (1) POTE polypeptide preparation: after prokaryotically expressed GST-POTE-144 fragment (GST-144) is affinity-bound with Glutathione-Sepharose beads, it is incubated with precision enzyme, The GST tag linked to the 144 polypeptide was excised, and then the digestion mixture was centrifuged at 14,000 rpm with a 10kDa pore size ultrafiltration tube, and the precision enzyme was removed by ultrafiltration to obtain a purified POTE-144 (POTE-E-144) polypeptide fragment. In the same way, POTE-390 (390-524aa) a...
Embodiment 3
[0077] Embodiment 3, the identification of POTE autoantibody epitope
[0078] The POTE autoantibodies purified from the serum of tumor patients by GST-144 affinity chromatography may be polyclonal antibodies against different sequence epitopes in the 1-144aa fragment polypeptide of POTE. In order to identify the epitope of the POTE autoantibody, in this example, the possible regional epitope within the 144 amino acid residues of the N-terminal of the POTE-E protein, that is, the 20th-53aa (SEQ ID No.2), was respectively encoded by PCR method The gene sequences corresponding to the 57-90aa (SEQ ID No.3) and 94-127aa (SEQ ID No.4) regions were cloned into the pGEX-6P-3 vector to construct pGEX-GST-POTE_20-53, _57- 90 and _94-127aa fusion protein expression vector. The above plasmids were transformed into Rosetta Escherichia coli, and IPTG induced the expression of the prokaryotic protein, namely the corresponding fragment protein of POTE-E. Escherichia coli was disrupted and l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com