Chiral supermolecule hydrogel and preparation method and application thereof
A supramolecular hydrogel and chiral technology, applied in the field of medicine or tissue engineering, can solve the problems of no biological activity, expensive preparation cost, pathogenicity, etc., and achieve easy mass industrial production, convenient operation, dispersion good sex effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
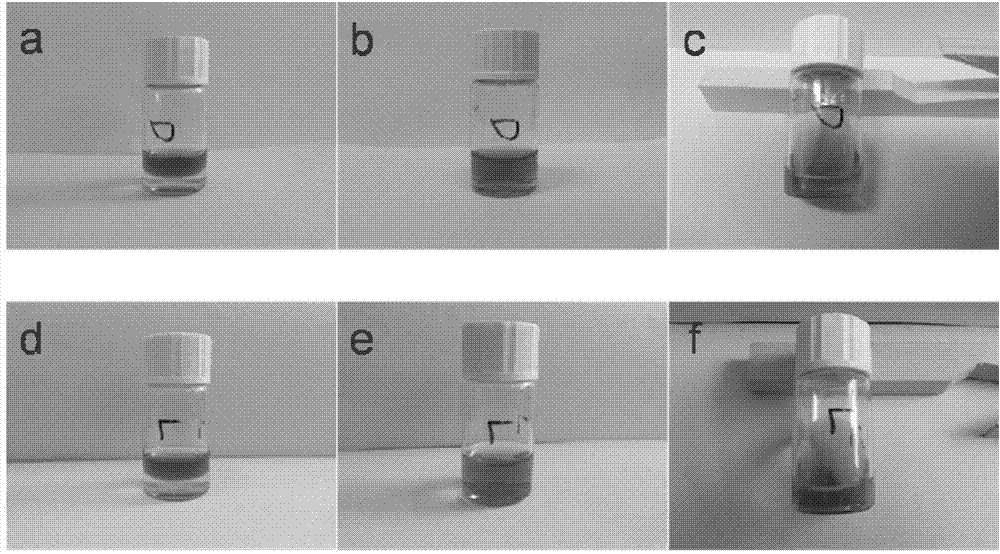
[0036] This embodiment relates to a chiral supramolecular hydrogel, the chiral supramolecular hydrogel is a phenylalanine derivative with a 1,4-benzene ring as the nucleus, and the structural formula of the derivative is as follows:
[0037]
[0038] Among them, R 1 for H
[0039] R 2 is COOEtOEtOH.
[0040] This embodiment also relates to the preparation method of the aforementioned chiral supramolecular hydrogel, the method comprising:
[0041] Step 1, get terephthaloyl chloride, L-phenylalanine methyl ester hydrochloride, triethylamine; The ratio is 1:2:6; the raw materials can be obtained from open commercial channels;
[0042] Step 2: Cool 10-40 mL of anhydrous dichloromethane solution of L-phenylalanine methyl ester hydrochloride to 0°C, add triethylamine, and then add 5-10 mL of terephthaloyl chloride to obtain a reaction solution ; The reaction solution was slowly raised to room temperature and stirred for 12 to 16 hours;
[0043] Step 3: Rotate the reaction s...
Embodiment 2
[0048] This embodiment relates to a chiral supramolecular hydrogel, the chiral supramolecular hydrogel is a phenylalanine derivative with a 1,4-benzene ring as the nucleus, and the structural formula of the derivative is as follows:
[0049]
[0050] Among them, R 1 is COOEtOEtOH,
[0051] R 2 for H.
[0052] This embodiment also relates to the preparation method of the aforementioned chiral supramolecular hydrogel, the method comprising:
[0053] Step 1, get terephthaloyl chloride, L-phenylalanine methyl ester hydrochloride, triethylamine; The ratio is 2:3:4; the raw materials can be obtained from open commercial channels;
[0054] Step 2: Cool 10 mL of anhydrous dichloromethane solution of L-phenylalanine methyl ester hydrochloride to 0° C., add triethylamine, and then add 5 mL of terephthaloyl chloride to obtain a reaction solution; Slowly warm to room temperature and stir for 12 hours;
[0055] Step 3: Rotate the reaction solution, add 10 mL of deionized water, an...
Embodiment 3
[0060] This embodiment relates to a chiral supramolecular hydrogel, the chiral supramolecular hydrogel is a phenylalanine derivative with a 1,4-benzene ring as the nucleus, and the structural formula of the derivative is as follows:
[0061]
[0062] Among them, R 1 is COOEtOEtOH,
[0063] R 2 for H.
[0064] This embodiment also relates to the preparation method of the aforementioned chiral supramolecular hydrogel, the method comprising:
[0065] Step 1, get terephthaloyl chloride, L-phenylalanine methyl ester hydrochloride, triethylamine; The ratio is 2:3:4; the raw materials can be obtained from open commercial channels;
[0066] Step 2: Cool 10 mL of anhydrous dichloromethane solution of L-phenylalanine methyl ester hydrochloride to 0° C., add triethylamine, and then add 5 mL of terephthaloyl chloride to obtain a reaction solution; Slowly warm to room temperature and stir for 12 hours;
[0067] Step 3: Rotate the reaction solution, add 10 mL of deionized water, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com