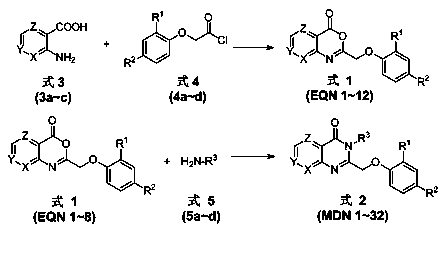
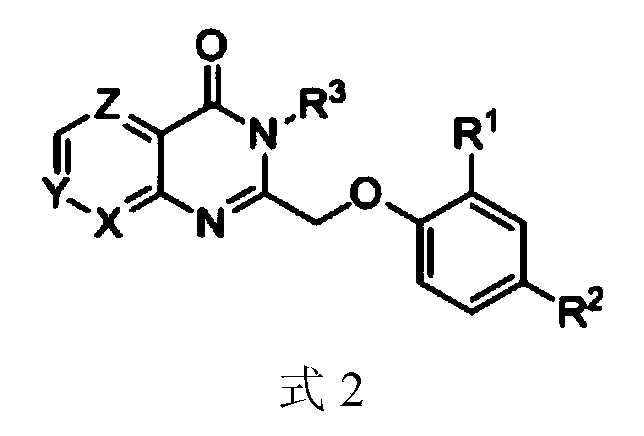
Pyridinooxazone-pyridinopyrimidone compounds and preparation method and application thereof
The technology of a compound and oxazinone, which is applied in the application field of weed control, can solve problems such as bio-ecological safety problems, and achieve the effects of good herbicidal activity, novel structure, and growth inhibition.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Synthesis of various phenoxyacetyl chlorides (Formula 4)
[0034]
[0035] The phenoxyacetyl chloride (formula 4) in the present invention is prepared from the corresponding substituted phenoxyacetic acid by a conventional acid chloride method. The general method of its synthesis is as follows: Dissolve phosphorus pentachloride in dichloromethane, add substituted phenoxyacetic acid under ice bath, heat and reflux reaction in oil bath for 10h, until no gas is released, remove dichloromethane by normal pressure distillation, and distill under reduced pressure Phosphorus oxychloride is removed to obtain a brownish-yellow liquid, which is substituted phenoxyacetyl chloride (Formula 4).
Embodiment 2
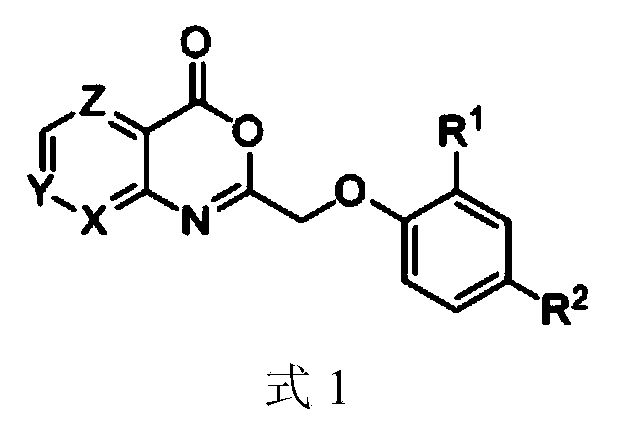
[0037] Preparation of 2-(2,4-dichlorophenoxymethyl)-4H-pyridin[2,3-d][1,3]oxazin-4-one (EQN1)
[0038]
[0039] Dissolve 2-aminonicotinic acid (3a, 0.3g, 2.2mmol) in 10mL DMF solution, add dropwise 2,4-dichlorophenoxyacetyl chloride (4a, 0.35mL, 2.2mmol) under ice-cooling, add Triethylamine (0.32mL, 2.2mmol), continue to react at room temperature for 12h. Filtered, washed with saturated saline and sodium bicarbonate solution successively, the organic layer was dried over anhydrous sodium sulfate, and then silica gel column chromatography was obtained to obtain light yellow solid 2-(2,4-dichlorophenoxymethyl)-4H-pyridine [2,3-d][1,3]oxazin-4-one (EQN1), yield 75%, m.p.198.8-199.6°C.
[0040] 1 H NMR(600MHz,DMSO-d6):δ9.03(d,J=3.0Hz,1H),8.56(d,J=7.2Hz,1H),7.56(q,J=7.2Hz,1H),7.40( s,1H),7.18(d,J=6.6Hz,1H),7.05(d,J=8.4Hz,1H),5.10(s,2H).EI-MSm / z,(%):321.93(M + ,1.03),286.96(100.00).
Embodiment 3
[0042] Preparation of 2-(4-chlorophenoxymethyl)-4H-pyridin[2,3-d][1,3]oxazin-4-one (EQN2)
[0043]
[0044] The compound 2-(4-chlorophenoxymethyl)-4H-pyridin[2,3-d][1,3]oxazin-4-one (EQN2) was obtained by a synthetic method similar to Example 2, light yellow Solid, yield 70%, m.p.184.5-185.5°C.
[0045] 1 H NMR (400MHz, CDCl 3 ):δ9.03(d,J=2.8Hz,H),8.55(d,J=6.4Hz,H),7.56( q,J=3.2Hz,H),7.25(d,J=2.0Hz,2H),7.01(d,J=2.4Hz,2H),5.07(s,2H).EI-MS m / z,(%) :287.98(M + ,47.98), 146.93(100.00).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Yield | aaaaa | aaaaa |
| Yield | aaaaa | aaaaa |
| Yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com