Multilayer bionic intestinal stent and preparation method thereof
A technology with a three-layer structure and intestinal tract, which is applied in the direction of stents, drug devices, and other medical devices, can solve the problems of secondary intestinal damage and high stress, and achieve simple operation, shortened operation time, easy learning and The effect of promotion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] 1. Preparation of scaffolds by knitting method;
[0065] 1) Bracket material;
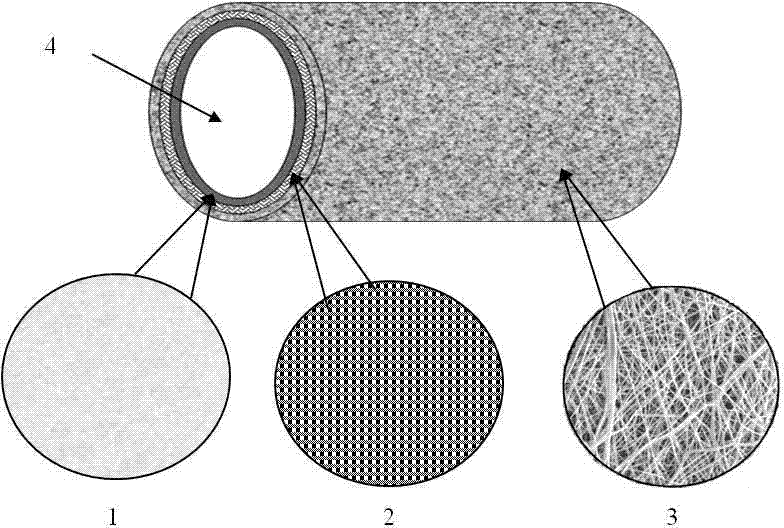
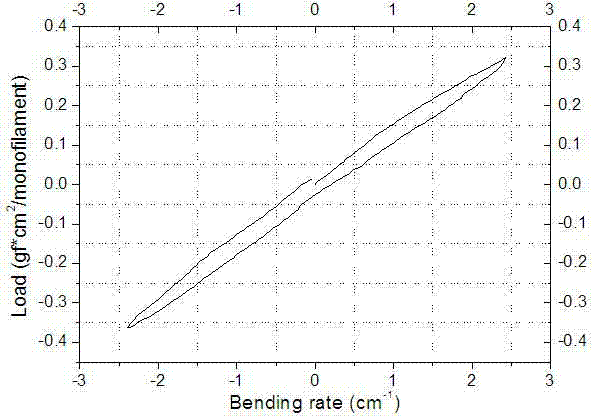
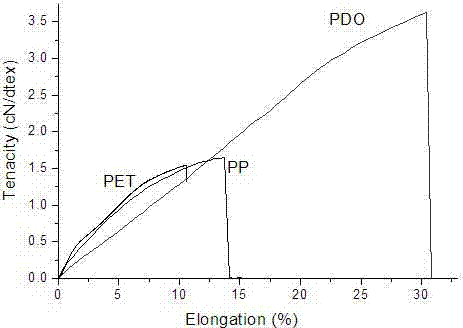
[0066] A medical stent raw material, polymer monofilament, high modulus, strength, monofilament diameter about 0.1-0.6mm, tensile strength greater than 400N / mm 2 , The breaking strength is greater than 600MPa, the melting temperature is higher than 90°C, and the breaking elongation is less than 40%. The selected materials are polyglycolic acid, polyglycolic acid, polydioxanone, and polylactic acid-glycolic acid copolymer. Such as figure 2 , 3 , 4, the selected medical grade polymer in this embodiment has good flexural rigidity performance, and the maximum flexural rigidity is 0.8gf*cm 2 The maximum breaking strength reaches 37N, and the breaking elongation is 45%. The plastic performance test of the selected absorbable polymer monofilament shows that before breaking, the material has no obvious plastic deformation point and has good resistance to plastic deformation.
[0067] 2) Molding...
Embodiment 2
[0074] 1. Preparation of scaffolds by braiding method;
[0075] 1) Bracket material;
[0076] A medical stent raw material, polymer monofilament, high modulus, strength, monofilament diameter about 0.1-0.6mm, tensile strength greater than 400N / mm2, breaking strength greater than 600MPa, melting temperature higher than 90°C, elongation at break less than 40%, and the bioabsorption period is greater than 60 days. The selected materials are polyglycolic acid, polyglycolic acid, polydioxanone, polylactic acid-glycolic acid copolymer. Such as figure 2 , 3 , 4, the selected absorbable polymer in this embodiment has good flexural rigidity, the maximum flexural rigidity is 0.8gf*cm2; the maximum breaking strength reaches 37N, and the breaking elongation is 45%; the selected absorbable polymer The plasticity test of the silk shows that before breaking, the material has no obvious plastic deformation point and has good resistance to plastic deformation.
[0077] 2) Molding process;...
Embodiment 3
[0084] 1. Prepare the scaffold by weaving method;
[0085] 1) Bracket material;
[0086] A medical stent raw material, polymer monofilament, high modulus, strength, monofilament diameter about 0.1-0.6mm, tensile strength greater than 400N / mm 2 , The breaking strength is greater than 600MPa, the melting temperature is higher than 90°C, the elongation at break is less than 40%, and the bioabsorption period is greater than 90 days. The selected materials are polyglycolic acid, polyglycolic acid, polydioxanone, and polylactic acid-glycolic acid copolymer. Such as figure 2 , 3 , As shown in 4, the selected medical polymer has good flexural rigidity performance in this embodiment, and the maximum flexural rigidity is 0.8gf*cm 2 The maximum breaking strength reaches 37N, and the breaking elongation is 45%. The plastic performance test of the selected absorbable polymer monofilament shows that before breaking, the material has no obvious plastic deformation point and has good resi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com