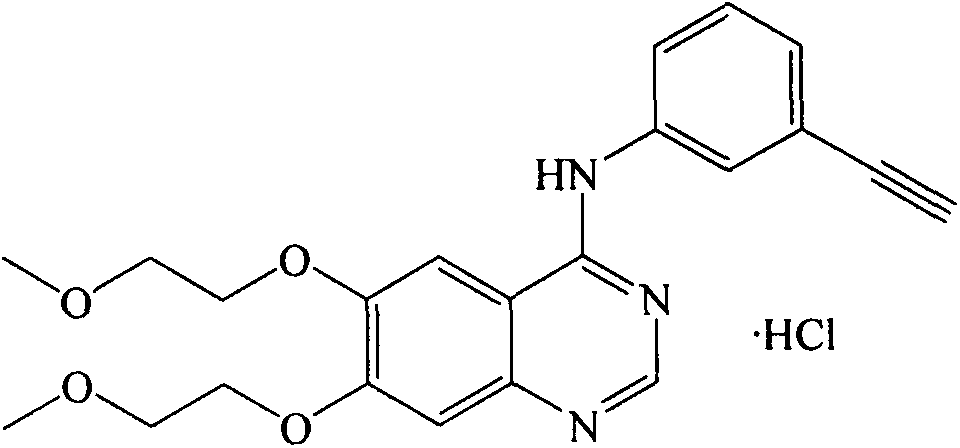
Tablets containing erlotinib hydrochloride and preparation method thereof
A technology of erlotinib hydrochloride tablets and erlotinib hydrochloride, which is applied in the field of medicine, can solve the problems that the dissolution rate is easily affected by temperature and humidity, inhomogeneity, and incomplete dissolution of active ingredients, etc., and achieves fast dissolution rate and Stability, improved dissolution rate, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0038] According to the prescription and process in the patent CN103110597A reference example 6, preparation sample preparation is carried out;
[0039] Preparation Process:
[0040] A, mixing materials except magnesium stearate (erlotinib hydrochloride micronized treatment, D90 is 5.7 μ m);
[0041] B, carry out dry granulation to the material obtained in step A;
[0042] C, add magnesium stearate and mix, and compress into tablet;
[0043] D, adopt the coating powder of trade name Opadry to carry out coating tablet.
Embodiment 1
[0044] Embodiment 1 (1000 pieces)
[0045]
[0046] A, prescription amount Erlotinib hydrochloride (crossing 80 mesh sieves) and silicon dioxide (crossing 100 mesh sieves), mix;
[0047] B. In the mixture of step A, add lactose, microcrystalline cellulose, sodium carboxymethyl starch, and a small amount of magnesium stearate respectively passing through a 100-mesh sieve of the prescription amount, and mix well;
[0048] C. Carry out dry granulation of the mixture obtained in the above step B, and granulate into 12 meshes;
[0049] D, the granule obtained in step C is mixed with the magnesium stearate of the remaining prescription amount, and compressed into tablets;
[0050] E, adopt the coating powder of trade name Opadry to coat tablet.
[0051] Embodiment 2 (1000 pieces)
[0052]
[0053] A, the erlotinib hydrochloride (crossing 200 mesh sieves) and silicon dioxide (crossing 100 mesh sieves) of prescription quantity micronized, mix;
[0054]B. In the mixture of st...
Embodiment 3
[0058] Embodiment 3 (1000 pieces)
[0059]
[0060] A, prescription amount Erlotinib hydrochloride and silicon dioxide are crossed 100 mesh sieves respectively, mix;
[0061] B. In the mixture of step A, add lactose, microcrystalline cellulose, sodium carboxymethyl starch, and a small amount of magnesium stearate respectively passing through a 100-mesh sieve of the prescription amount, and mix well;
[0062] C. The mixture obtained in the above step B is subjected to dry granulation, and granulated at 30 mesh;
[0063] D, the granule obtained in step C is mixed with the magnesium stearate of the remaining prescription amount, and compressed into tablets;
[0064] E, adopt the coating powder of trade name Opadry to coat tablet.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com