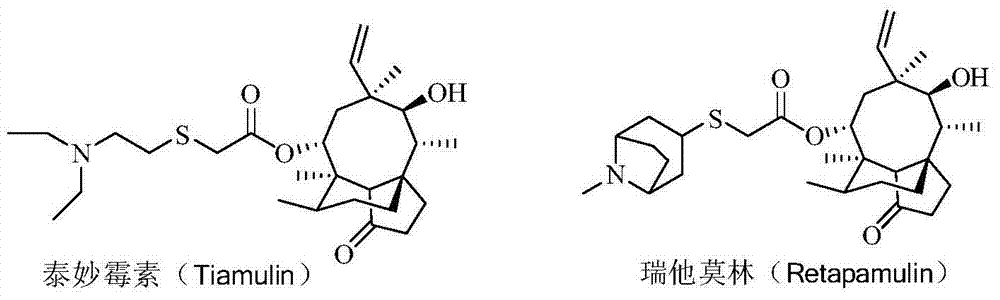
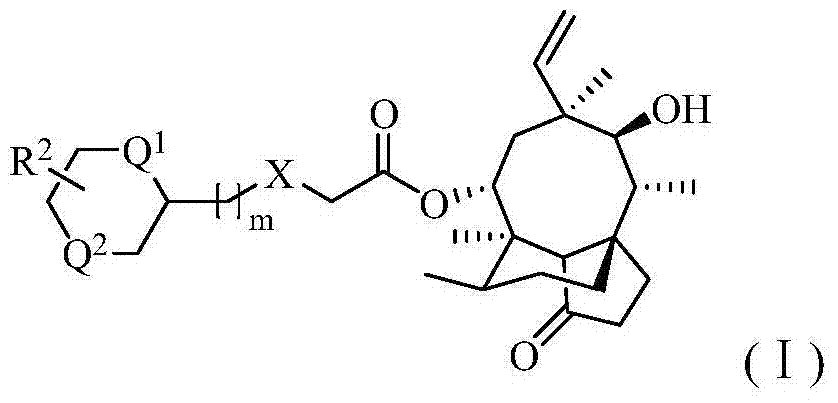
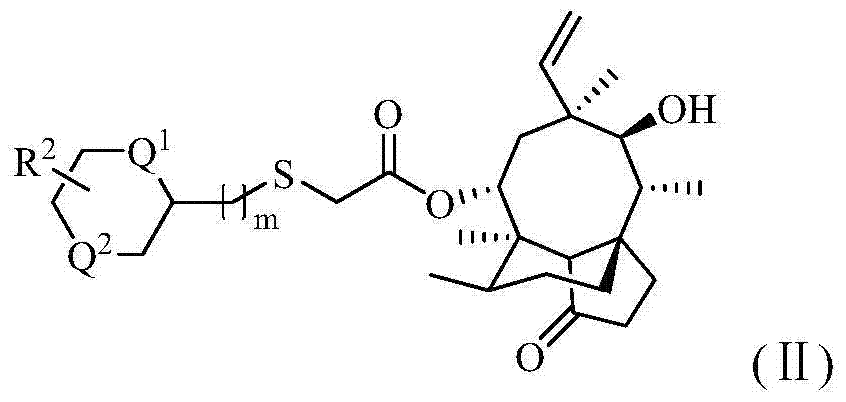
Pleuromutilin antibiotic derivatives
A compound and prodrug technology, applied in the field of pleuromutilin antibiotic derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 14
[0240] The preparation of embodiment 14-O-(p-toluenesulfonylacetyl) tiamulin (intermediate M1)
[0241]
[0242] Tiamulin (SM1, 37.8g, 0.1mol) was dissolved in 60ml of dichloromethane, and triethylamine (15.15g, 0.15mol) was added under nitrogen protection. Stir for 30 minutes, cool down to -15°C, and add dropwise TosCl (13.75 g, 0.12 mol) into a dichloromethane solution. The mixture was stirred for 2 hours, and 1.15 L of water was added slowly. The organic phase was separated, dried, and spin-dried to obtain the product intermediate M1 (44g, 96%).
[0243] With reference to the above preparation method, the following compounds can also be prepared:
[0244]
Embodiment 114
[0245] Example 114-O-(4-Methylmorpholin-2-yl)methylthio)Tiamulin (Compound 1) Preparation
[0246]
[0247] (1) Preparation of 14-O-((N-Boc-4-methylmorpholin-2-yl)methylthio)tiamulin
[0248]
[0249] 22-O-methylsulfonyl tiamulin (3g, 6.369mmol), N-Boc-2-mercaptomethylmorpholine (3.0g, 12.2mmol), NaOH aqueous solution (1N, 1.12g) and triethyl Benzyl ammonium chloride (300mg, 1.4mmol) was dissolved in 100mL MTBE, reacted overnight at 90°C under nitrogen protection, concentrated, added water, extracted with ethyl acetate, dried, concentrated and separated by silica gel column (petroleum ether: ethyl acetate =5:1) 3.0 g of the product was obtained with a yield of 79%.
[0250] (2) Preparation of 14-O-((4-methylmorpholin-2-yl)methylthio)tiamulin trifluoroacetate
[0251]
[0252] Dissolve 14-O-((N-Boc-4-methylmorpholin-2-yl)thio)tiamulin (3.0 g, 5.0 mmol) in 100 mL of DCM, add 20 mL of trifluoroacetic acid, and After reacting for 3 h, it was concentrated to obtain an...
Embodiment 214
[0258] Example 214-O-(1-methyl-4-((2R)-2-amino-3-methylbutyryl)piperazin-2-yl)methylthio)tiamulin (compound 2 ) preparation
[0259]
[0260] (1) 14-O-((N-Boc-1-methyl-4-((2R)-2-amino-3-methylbutyryl)piperazin-2-yl)methylthio)tai Preparation of myocin
[0261]
[0262] 22-O-methylsulfonyl tiamulin (1.0g, 2.2mmol), N-Boc-3-mercaptomethyl-4-methylpiperazine (1.23g, 5mmol), 5mL1N NaOH aqueous solution and triethyl Benzyl ammonium chloride (0.1g, 0.44mmol) was dissolved in 000mL dioxane, reacted overnight at 90°C under nitrogen protection, concentrated, added water, extracted with ethyl acetate, dried, concentrated, and separated by silica gel column (petroleum ether : ethyl acetate=10:1) to obtain product 2g, productive rate 60%.
[0263] (2) 14-O-((1-methyl-4-((2R)-2-amino-3-methylbutyryl)piperazin-2-yl)methylthio)tiamulin tri Preparation of fluoroacetate
[0264]
[0265]14-O-((N-Boc-1-methyl-4-((2R)-2-amino-3-methylbutyryl)piperazin-2-yl)methylthio)Tiamulina T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com