Transition metal compounds based on semi-rigid, synthetic method and applications
A technology of methyl isophthalic acid and bispyridine bisamide, applied in the direction of organic compound/hydride/coordination complex catalysts, chemical instruments and methods, cobalt organic compounds, etc., can solve the problem of poor catalytic degradation effect and catalytic ability Limitations, poor affinity for organic pollutants, etc., to achieve the effect of increasing flexibility, good catalytic performance, and increasing hydrophilicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Example 1 Synthesis of [Cu(3-bpah)(5-MIP)]·2H 2 O, where 3-bpah is N , N '-bis(3-pyridinecarboxamide)-1,2-cyclohexane, the structural formula is: , 5-MIP is 5-methylisophthalic acid
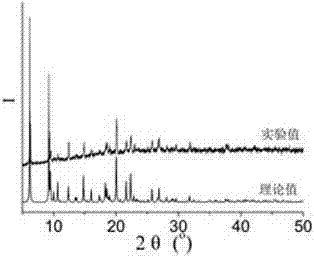
[0072] 0.1mmol CuCl 2 2H 2 O, 0.10mmol N , N '-bis(3-pyridinecarboxamide)-1,2-cyclohexane, 0.10mmol 5-methylisophthalic acid and 7.8mL H 2 O was sequentially added to a 25mL beaker, and stirred at room temperature for 30min to obtain a suspension mixture. After the pH of the suspension mixture was adjusted to 6.4 with 0.1mol / L NaOH solution, it was transferred to a 25mL autoclave. The heating rate was increased to 130°C, and the temperature was kept for 48 hours under hydrothermal conditions, and the temperature was lowered to room temperature at a cooling rate of 5°C / h to obtain blue blocky crystals, which were washed alternately with deionized water and ethanol for 3 times, and naturally dried at room temperature Dry to get [Cu(3-bpah)(5-MIP)]·2H 2 O, the productive rate is 30%...
Embodiment 2
[0073] Example 2 Synthesis of [Cu(3-bpah)(5-MIP)]·2H 2 O, where 3-bpah is N , N '-bis(3-pyridinecarboxamide)-1,2-cyclohexane, 5-MIP as 5-methylisophthalic acid
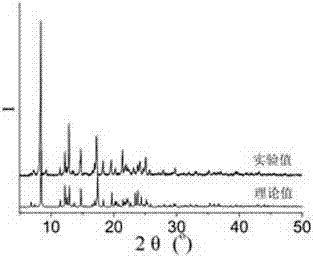
[0074] 0.1mmol CuCl 2 2H 2 O, 0.10mmol N , N '-bis(3-pyridinecarboxamide)-1,2-cyclohexane, 0.15mmol 5-methylisophthalic acid and 8.0mL H 2O was sequentially added to a 25mL beaker, and stirred at room temperature for 40min to obtain a suspension mixture. After adjusting the pH of the suspension mixture to 6.7 with 0.1mol / L NaOH solution, it was transferred to a 25mL autoclave and heated at 15°C / h Raise the temperature to 110°C, keep it warm for 96 hours under hydrothermal conditions, then lower the temperature to room temperature at a cooling rate of 5°C / h to obtain blue blocky crystals, wash them alternately with deionized water and ethanol for 5 times, and dry them naturally at room temperature , to get [Cu(3-bpah)(5-MIP)]·2H2O, the productive rate is 33%, and its XRD diffraction pattern is as figure 1 As s...
Embodiment 3
[0075] Example 3 Synthesis of [Cu(3-bpah)(5-MIP)]·2H 2 O, where 3-bpah is N , N '-bis(3-pyridinecarboxamide)-1,2-cyclohexane, 5-MIP as 5-methylisophthalic acid
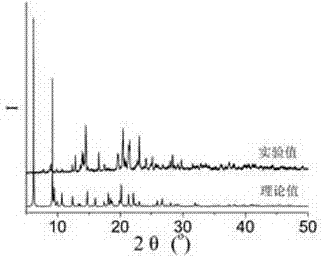
[0076] 0.2mmol CuCl 2 2H 2 O, 0.10mmol N , N '-bis(3-pyridinecarboxamide)-1,2-cyclohexane, 0.2mmol 5-methylisophthalic acid and 8.0mL H 2 O was sequentially added to a 25mL beaker, and stirred at room temperature for 20min to obtain a suspension mixture. After the pH of the suspension mixture was adjusted to 7.0 with 0.1mol / L NaOH solution, it was transferred to a 25mL autoclave. The heating rate was increased to 120°C, and the temperature was kept for 72 hours under hydrothermal conditions, and the temperature was lowered to room temperature at a cooling rate of 5°C / h to obtain blue blocky crystals, which were washed twice with deionized water and ethanol alternately, and naturally dried at room temperature Dry to get [Cu(3-bpah)(5-MIP)]·2H 2 O, the productive rate is 39%, and its XRD diffraction pattern is a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| catalytic efficiency | aaaaa | aaaaa |
| catalytic efficiency | aaaaa | aaaaa |
| catalytic efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com