Catalyst for preparing advanced hydrocarbon by Fischer-Tropsch synthesis and preparation method of catalyst
A technology for catalyst and Tropsch synthesis, which is applied in the Fischer-Tropsch synthesis catalyst and its preparation with high cobalt loading, and in the field of catalyst and its preparation. It can solve the problems of unsuitable long-chain hydrocarbon catalysts, restricting large-scale applications, and high price, so as to reduce the selection of CH4. performance, enhanced catalytic activity, and increased specific surface effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
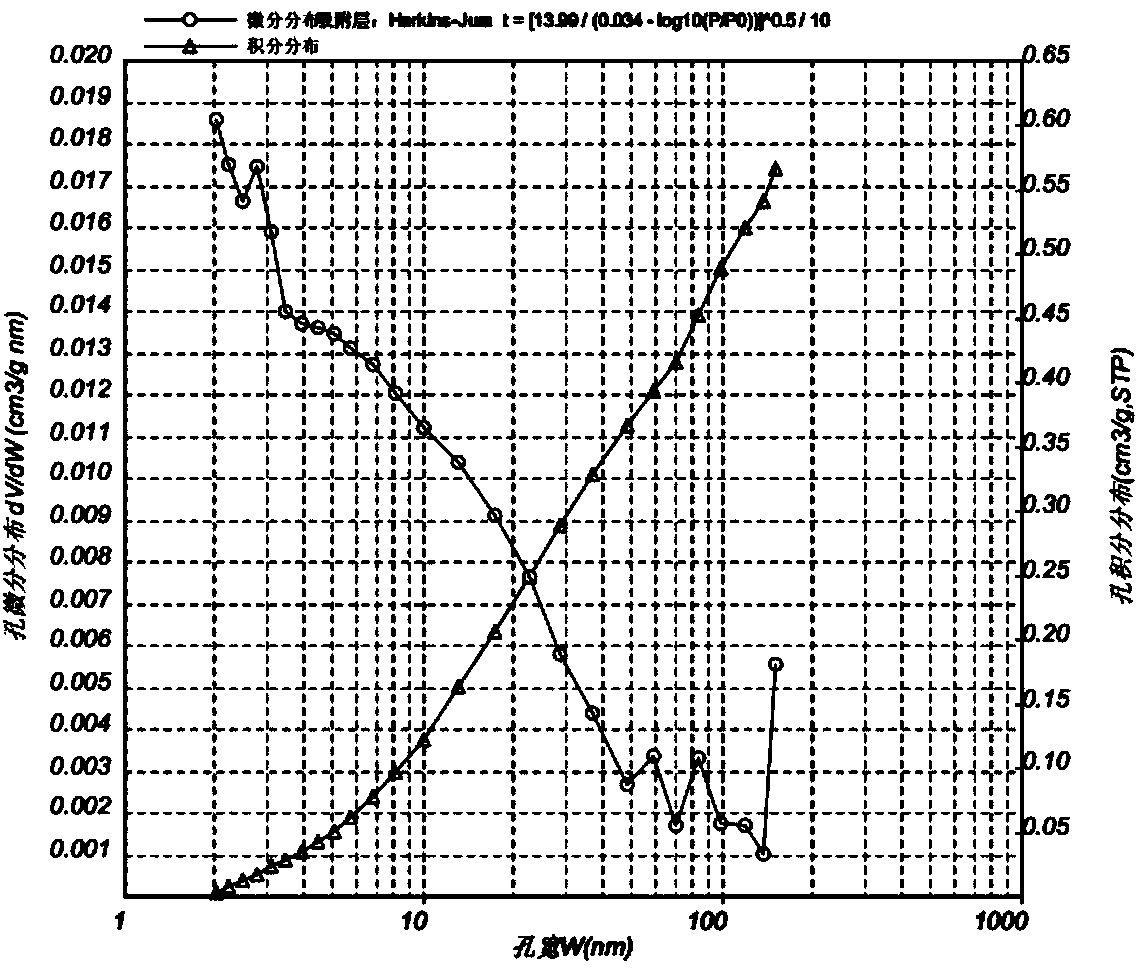
[0047] Weigh 21.69g Co(NO 3 ) 2 ·6H 2 O and 0.83g Zr(NO 3 ) 4 ·5H 2 O was dissolved in 60mL deionized water; another 5.55g Na 2 SiO 3 9H 2 O, 8.90g Na 2 CO 3 Dissolve in water to form a 60mL solution. Add the above two solutions dropwise to 100mL deionized water (80°C water bath) at the same time under stirring to form a purple precipitate, wash the precipitate with deionized water, filter it with suction and add it to the flask, and add KNO 3 0.247g and 0.221g of polyethylene glycol (molecular weight: 10,000) were dissolved in 5mL of deionized water and added to the flask, then 50mL of ethanol was added, and magnetically stirred for 2h. Distill the solvent in an oil bath at 110-120°C under stirring, take out the sample, and dry it at 110-120°C for 10 hours to obtain a cobalt-based Fischer-Tropsch synthesis catalyst precursor, wherein the cobalt content is 75wt%, and the zirconium content is 3wt%. The potassium content is 1.5 wt%. The pore size distribution diagram...
Embodiment 2
[0055] Weigh 18.80g Co(NO 3 ) 2 ·6H 2 O and 1.11g Zr(NO 3 ) 4 ·5H 2 O was dissolved in 60mL deionized water; another 8.12g Na 2 SiO 3 9H 2 O, 7.60g Na 2 CO 3 Dissolve in water to form a 60mL solution. Add the above two solutions dropwise to 100mL deionized water (80°C water bath) under stirring to form a purple precipitate, wash the precipitate with deionized water, filter it with suction and add it to the flask, and add KNO 3 0.165g and 0.221g of polyethylene glycol (molecular weight: 10,000) were dissolved in 5mL of deionized water and added to the flask, then 50mL of ethanol was added, and magnetically stirred for 2h. The solvent was distilled off in an oil bath at 110-120°C under stirring, the sample was taken out, and the cobalt-based Fischer-Tropsch synthesis catalyst precursor was obtained after drying, wherein the cobalt content was 65wt%, the zirconium content was 4wt%, and the potassium content was 1wt%.
Embodiment 3
[0059] Weigh 15.90g Co(NO 3 ) 2 ·6H 2 O and 0.55g Zr(NO 3 ) 4 ·5H 2 O was dissolved in 60mL deionized water; another 11.11g Na 2 SiO 3 9H 2 O, 6.60g Na 2 CO 3 Dissolve in water to form a 60mL solution. Add the above two solutions dropwise to 100mL deionized water (80°C water bath) under stirring to form a purple precipitate, wash the precipitate with deionized water, filter it with suction and add it to the flask, and add KNO 3 0.329g and 0.221g of polyethylene glycol (molecular weight: 10,000) were dissolved in 5mL of deionized water and added to the flask, then 50mL of ethanol was added, and magnetically stirred for 2h. Distill the solvent in an oil bath at 110-120°C under stirring, take out the sample, and dry it at 110-120°C to obtain a cobalt-based Fischer-Tropsch synthesis catalyst precursor, wherein the cobalt content is 55wt%, the zirconium content is 2wt%, and the potassium content is 2wt%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com