Beta-xylosidase in vivo enzyme aggregate and preparation method thereof
A technology of xylosidase and enzyme aggregates, applied in biochemical equipment and methods, glycosylases, enzymes and other directions, can solve problems such as no change, and achieve the effect of improving catalytic efficiency, promoting industrial application, and improving catalytic properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Construction of expression vectors containing encoding short peptides
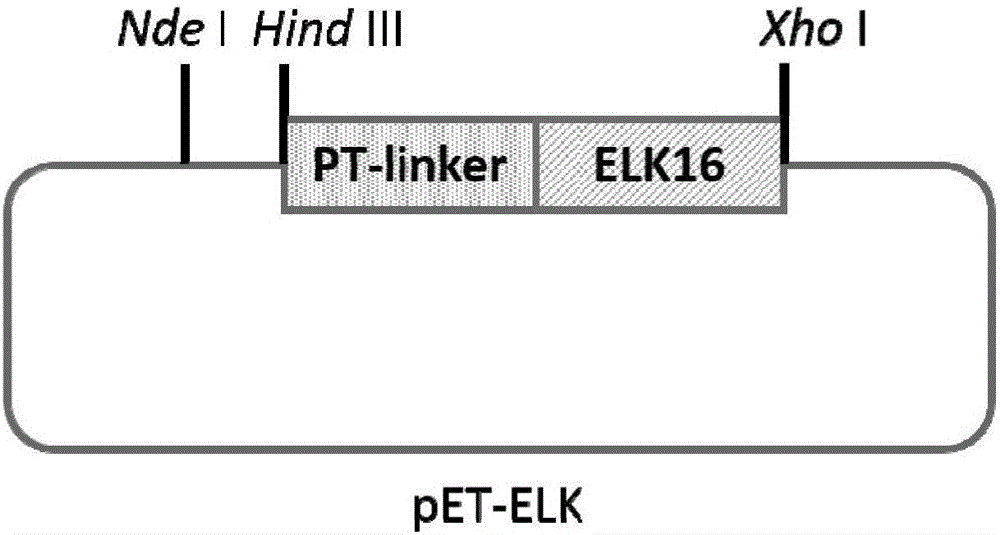
[0034] According to the amino acid sequence of ELK16 (LELELKLKLELELKLK, see SEQ ID: 2), a corresponding DNA sequence containing a stop codon was synthesized at a gene synthesis company. Among them, a PT-linker (PTPPTTPTPPTTPTPTP) sequence was introduced upstream of the ELK16 sequence, and HindIII and XhoI restriction sites were introduced at both ends of the synthetic sequence. The complete gene sequence is shown in SEQ ID: 1. Using the plasmid pET-30a(+) as the basic plasmid, it was digested with HindIII and XhoI, ligated into the corresponding digested ELK16 DNA sequence, and the ligated product was transformed into E.coli DH5α competent cells, and analyzed by colony PCR, restriction enzyme digestion and sequencing Positive transformants were identified to obtain the expression vector pET-ELK, such as figure 1 shown.
Embodiment 2
[0036] Construction of β-xylosidase expression strain
[0037] 1. Amplification of the β-xylosidase gene
[0038] Using the genomic DNA of Thermoanaerobacterium aotearoense SCUT27 as a template, the β-xylosidase gene (GenBank: KX372717) was amplified, and the PCR primers used were as follows:
[0039] XylC-F: 5′-TCGGCT CATATG GAATACCATGTGGCTAAAA-3' (see SEQ ID: 3), the underlined base is the NdeI restriction site.
[0040] XylC-R: 5′-TAGCAA CTCGAG AGAAGAGCCCCAAACTTTTATGTAATTATTTCCT-3' (see SEQ ID: 4), the underlined base is the XhoI restriction site.
[0041] XylC-E-R: 5'-CTGTTC AAGCTT CCAAACTTTTTATGTAATTATTTCCT-3' (see SEQ ID: 5), the underlined base is the HindIII restriction site.
[0042] The primer pairs XylC-F / XylC-R and XylC-F / XylC-E-R were amplified respectively to obtain β-xylosidase genes xylC1 and xylC2 with a length of about 2 kb.
[0043] 2. Construction of recombinant expression vector
[0044] The purified PCR products xylC1 and pET30a(+) were double-d...
Embodiment 3
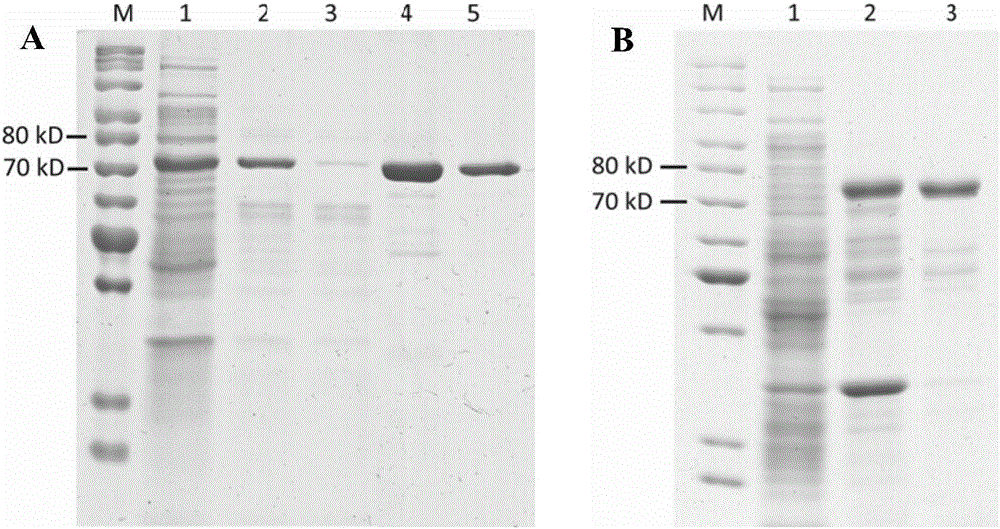
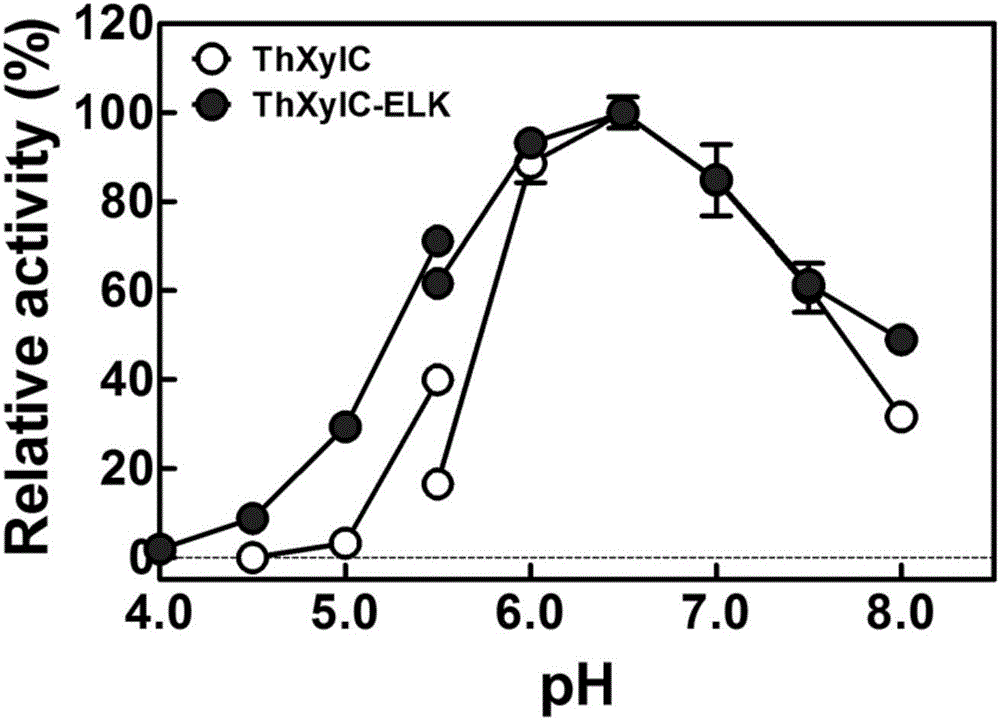
[0047] Obtainment of routinely expressed soluble β-xylosidase (ThXylC) and active β-xylosidase aggregates (ThXylC-ELK16).
[0048] The overnight cultured recombinant engineered bacteria E.coli BL21(DE3) / pET-xylC1 and E.coli BL21(DE3) / pET30-xylC2-ELK were respectively inoculated at a ratio of 1:100 in fresh water containing 50 μg / ml kanamycin In LB liquid medium, culture at 37°C and 250rpm until the cell density OD 600 About 0.5-0.7, add IPTG with a final concentration of 1 mmol / L to induce the expression of β-xylosidase, and then continue to culture at 30° C. and 180 rpm for 24 hours.
[0049] The bacteria were collected by centrifugation at 4°C and 8000 g, and 10 ml of PB buffer (50 mmol / L phosphate buffer, 50 mmol / L sodium chloride, pH 7.5) was added to each 1 g of wet bacteria to resuspend, and the cells were sonicated. 15000g high-speed centrifugation for 20 minutes.
[0050] For E.coli BL21(DE3) / pET-xylC1, discard the precipitate after centrifugation, and first incubate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com