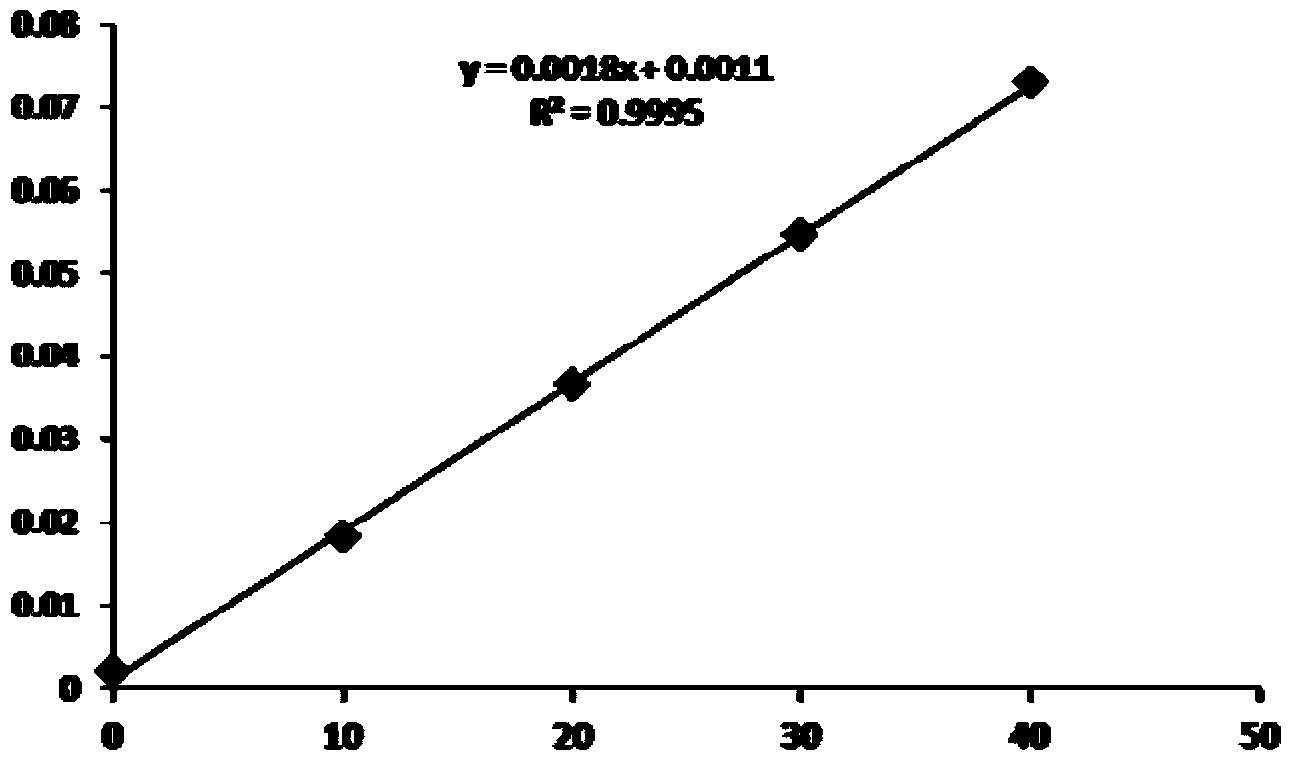
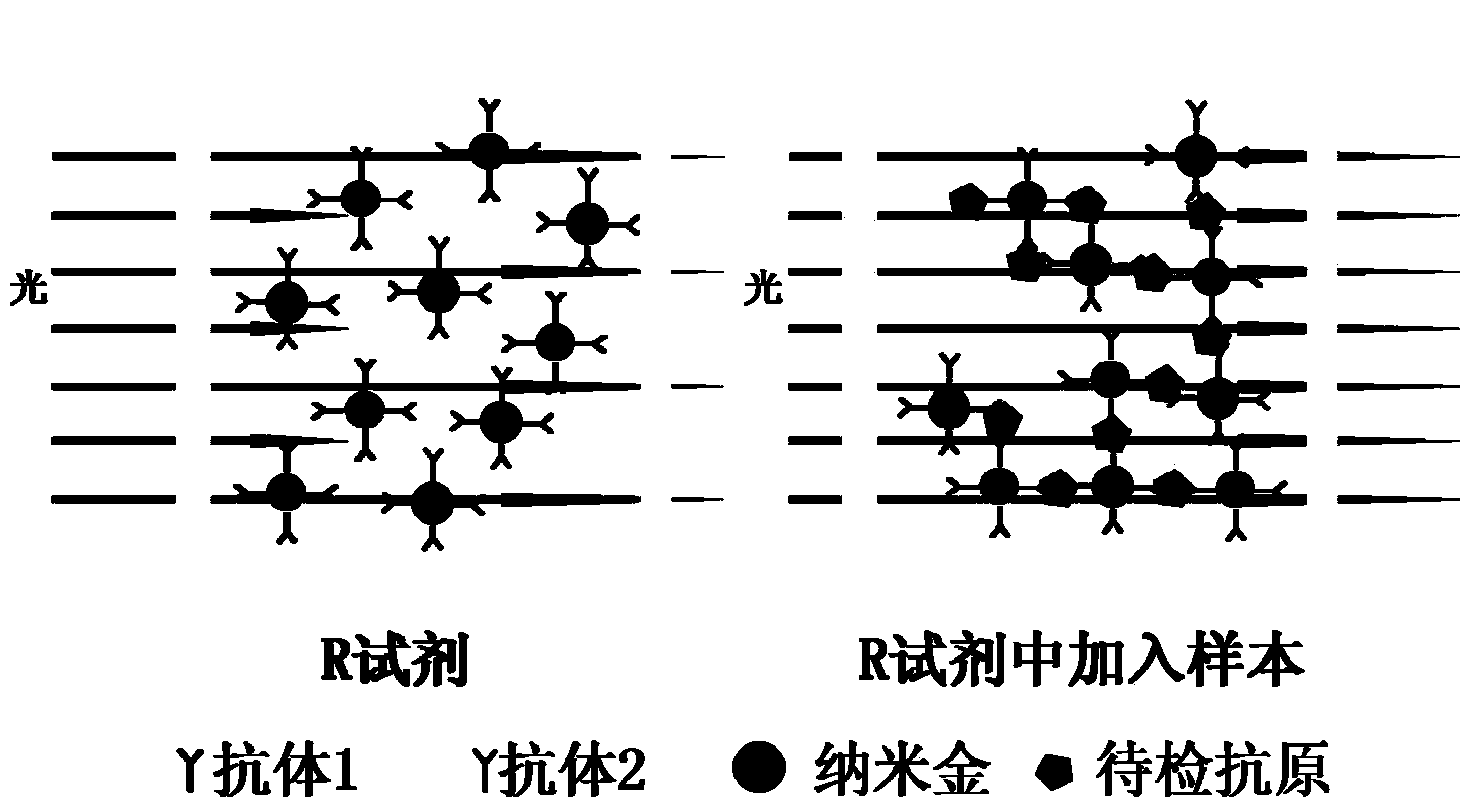
Extracorporal test kit for TAFI (Thrombin Activatable Fibrinolysis Inhibitor) content, and test method of extracorporal test kit
An in vitro detection and kit technology, which is applied in measurement devices, instruments, disease diagnosis, etc., can solve the problems of expensive equipment, long detection time, and low detection limit, and achieves simple operation, short detection time, and low detection limit. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] 1. Preparation of gold nanoparticles
[0032] The nano-gold used in the present invention is prepared by using sodium citrate and chloroauric acid. Firstly, the chloroauric acid solution is added with a small amount of nano-gold protective agent, then heated to boiling with an electric furnace, and trisodium citrate solution is added according to the mass ratio of 4:5. After continuing to heat for 10min, the existing nano-gold solution is calculated, and the volume of the nano-gold solution is theoretically prepared. After constant volume, utilize a nucleic acid protein analyzer or an ultraviolet spectrophotometer to carry out full-wavelength scanning to determine its peak value. The nano-gold solution used in the present invention The solution is scanned at full wavelength, and its peak is between 515-530nm.
[0033] 2. Preparation of monoclonal antibody and gold-labeled antibody
[0034] Monoclonal antibodies and gold-labeled antibodies are mouse anti-human monoclona...
Embodiment 1
[0051] Taking human plasma as an example, the method of the present invention is used to measure the TAFI content of 60 patients' plasma samples diagnosed as myocardial infarction and 300 healthy plasma samples. The specific operation steps are as follows:
[0052] Step 1 Collect human individual biological samples and immediately centrifuge at 4°C within 30 minutes to take the supernatant as the sample to be tested, and put it in an ice box for use within 1 hour or store it at -20°C for later use;
[0053] Step 2 uses an in vitro detection kit to measure the TAFI content of the sample to be tested;
[0054] The contents of the in vitro detection kit include: TAFI biochemical detection reagent R, TAFI standard substance, TAFI quality control substance, and instructions for use.
[0055] Step 3 Take out the reagent R and place it at room temperature until the temperature is balanced;
[0056] Step 4 put the R reagent and TAFI standard in the corresponding position of the bioc...
Embodiment 2
[0061] The method of the invention can also measure the TAFI content in human urine. Collect human morning urine, centrifuge at 1500 rpm for 5 minutes at 4°C within 30 minutes, and take the supernatant as the sample to be tested. Subsequent steps are the same as in Embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com