Oxcarbazepine tablets and preparation method thereof
A technology for oxcarbazepine tablets and oxcarbazepine tablets, which is applied in the field of tablet formulations of neurological drugs, can solve problems such as poor stability, and achieve the effects of less impurities, lower production costs, and shorter production cycles.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
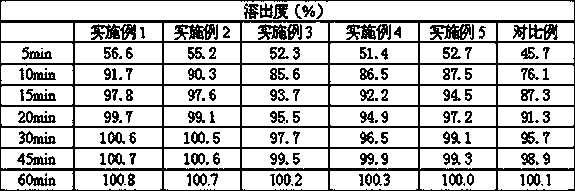
Examples
Embodiment 1
[0076] 10000 tablets product formula:
[0077] Oxcarbazepine raw material 1500g (accounting for 47.00% of the total weight of the tablet)
[0078] Partially pregelatinized starch 223g (accounting for 7.00% of the total weight of the tablet)
[0079] Starch lactose complex 957g (accounting for 30.00% of the total weight of the tablet)
[0080] S-630 copovidone 159g (accounting for 5.00% of the total weight of the tablet)
[0081] Croscarmellose Sodium 191g (6.00% of the total tablet weight)
[0082] Sodium stearyl fumarate 64g (accounting for 2.00% of the total weight of the tablet);
[0083] Coating (3 % of total tablet weight):
[0084] Hypromellose 41.2 g (43.00 % of coating weight)
[0085] Talc powder 34.5g (accounting for 36.00% of coating weight)
[0086] Titanium dioxide 9.6g (10.00% of coating weight)
[0087] Polyethylene glycol 4000 7.7g (accounting for 8.00% of coating weight)
[0088] Iron oxide yellow 2.9g (3.00% of coating weight).
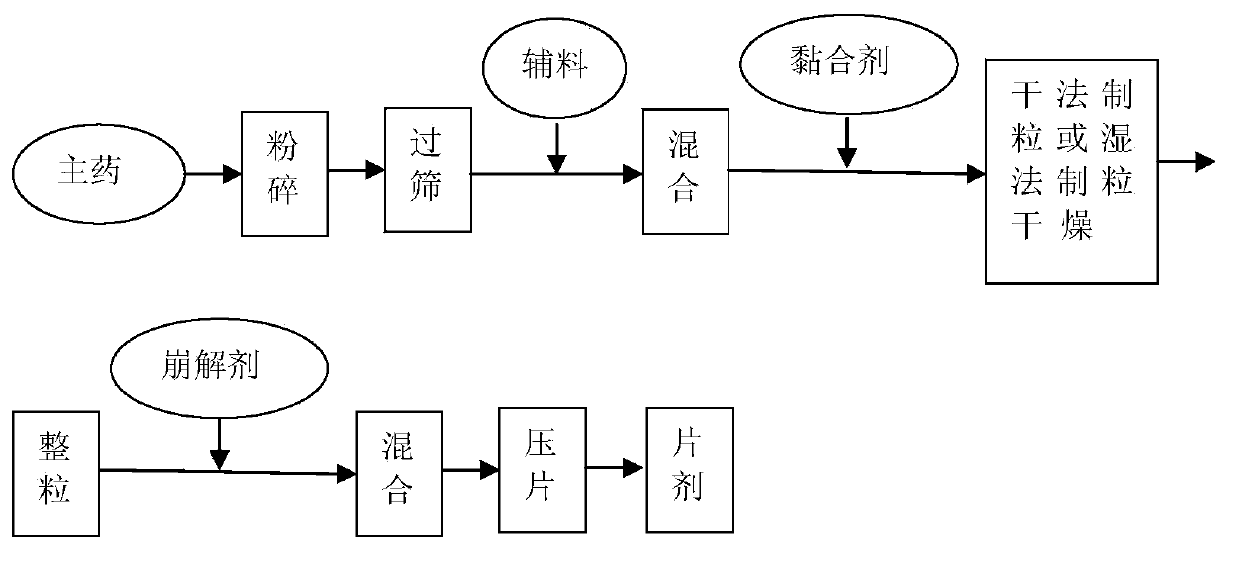
[0089] Preparation ...
Embodiment 2
[0092] 10000 tablets product formula:
[0093] Oxcarbazepine raw material 1500g (accounting for 55.00% of the total weight of the tablet)
[0094] Partially pregelatinized starch 136g (5.00% of the total weight of the tablet)
[0095] Starch lactose complex 764g (28.00% of the total weight of the tablet)
[0096] S-630 copovidone 109g (accounting for 4.00% of the total weight of the tablet)
[0097] Croscarmellose sodium 136g (5.00% of the total tablet weight)
[0098] Sodium stearyl fumarate 27g (accounting for 1.00% of the total weight of the tablet);
[0099] Coating: (2 % of total tablet weight)
[0100] Hypromellose 25.6 g (47.00 % of coating weight)
[0101] Talc powder 18.0g (33.00% of coating weight)
[0102] Titanium dioxide 4.9g (9.00% of coating weight)
[0103] Polyethylene glycol 4000 4.9g (9.00% of coating weight)
[0104] Iron oxide yellow 1.1g (2.00 % of coating weight).
[0105] Preparation Process:
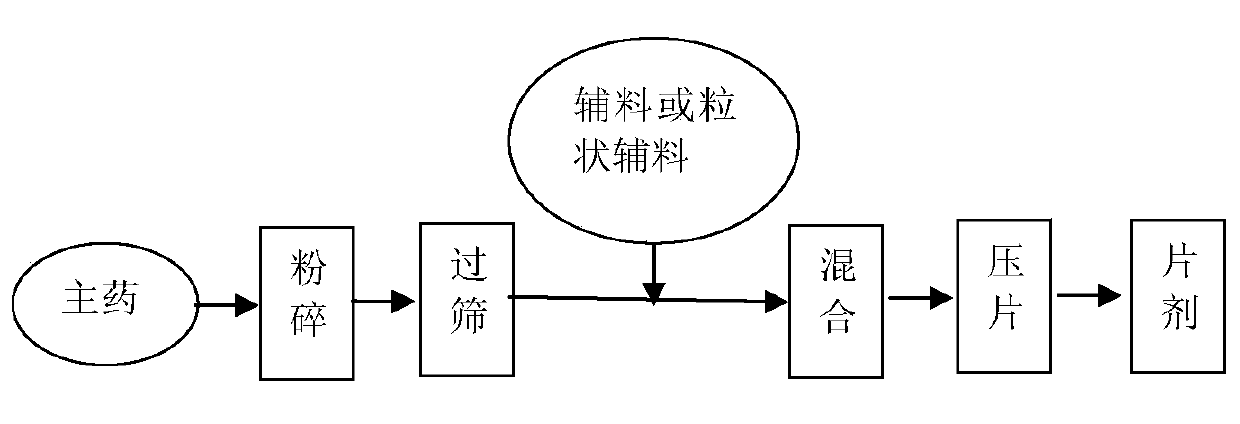
[0106] 1. Pass oxcarbazepine raw material, pa...
Embodiment 3
[0107] Embodiment 3: 10000 product formulas:
[0108] Oxcarbazepine raw material 1500g (accounting for 45.00% of the total weight of the tablet)
[0109] Partially pregelatinized starch 333g (10.00% of the total weight of the tablet)
[0110] Starch lactose complex 1067g (32.00% of the total weight of the tablet)
[0111] S-630 copovidone 100g (accounting for 3.00% of the total weight of the tablet)
[0112] Croscarmellose Sodium 200g (6.00% of the total tablet weight)
[0113] Sodium stearyl fumarate 83g (accounting for 2.50% of the total weight of the tablet);
[0114] Coating: (1.5 % of total tablet weight)
[0115] Hypromellose 25g (50.00 % of coating weight)
[0116] Talc powder 15g (accounting for 30.00% of coating weight)
[0117] Titanium dioxide 5.5g (11.00 % of coating weight)
[0118] Polyethylene glycol 4000 3.5g (7.00% of coating weight)
[0119] Iron oxide yellow 1g (2.00% of coating weight).
[0120] Preparation Process:
[0121]1. Pass oxcarbazepine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com