Method for making aluminum oxide by utilizing crystalline aluminum chloride
A technology for crystallizing aluminum chloride and aluminum chloride, which is applied in the preparation of alumina/hydroxide, etc., which can solve problems such as difficult high-purity alumina and increased impurity concentration, and achieve improved purity, reduced viscosity, and reduced energy consumption Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
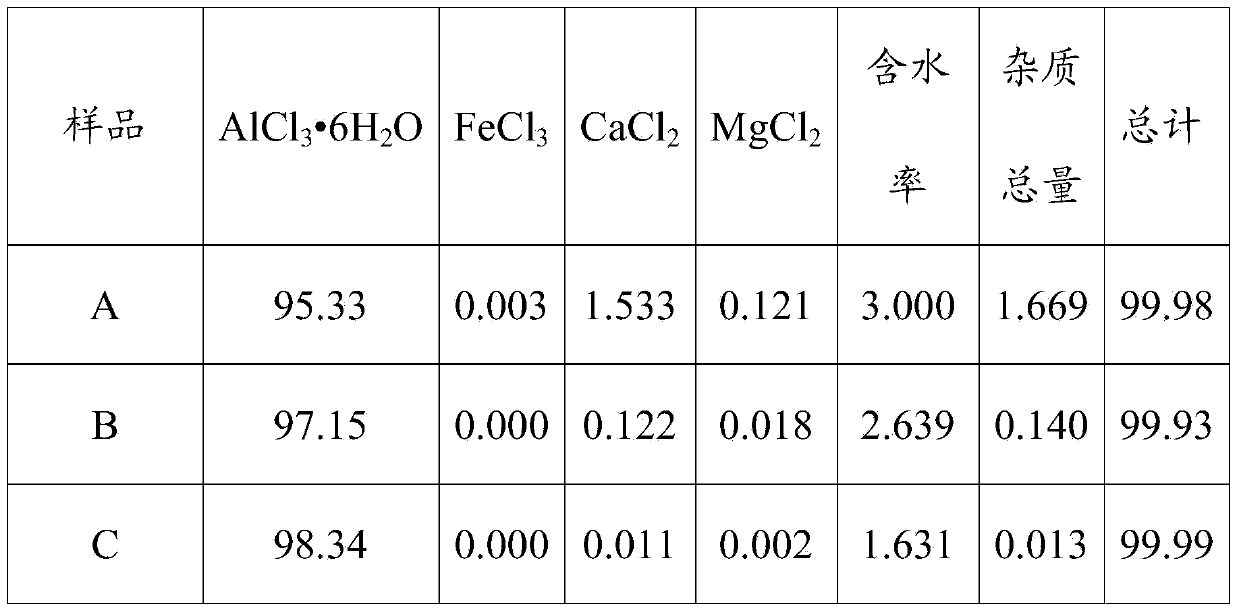
[0041] Add the above-mentioned crystalline aluminum chloride A gradually to 200ml of hydrochloric acid solution with a concentration of 16.73wt%, the operating temperature is 20°C, and keep stirring until the added crystalline aluminum chloride A is no longer dissolved, so as to obtain a saturated solution of aluminum chloride. The remaining crystalline aluminum chloride A to be purified is added to the saturated solution in an amount of 4:1 according to the liquid-solid ratio (that is, the mass ratio of the saturated aluminum chloride solution to the crystalline aluminum chloride A to be purified, the same below), Stir continuously to make it reach the equilibrium of dissolution and crystallization, and the stirring time is 60min to carry out the first purification; solid-liquid separation, to obtain a purified aluminum chloride crystal B, control the water content of the purified aluminum chloride crystal B at 2wt%~3 Between %wt; add 32wt% to 33wt% concentrated hydrochloric a...
Embodiment 2
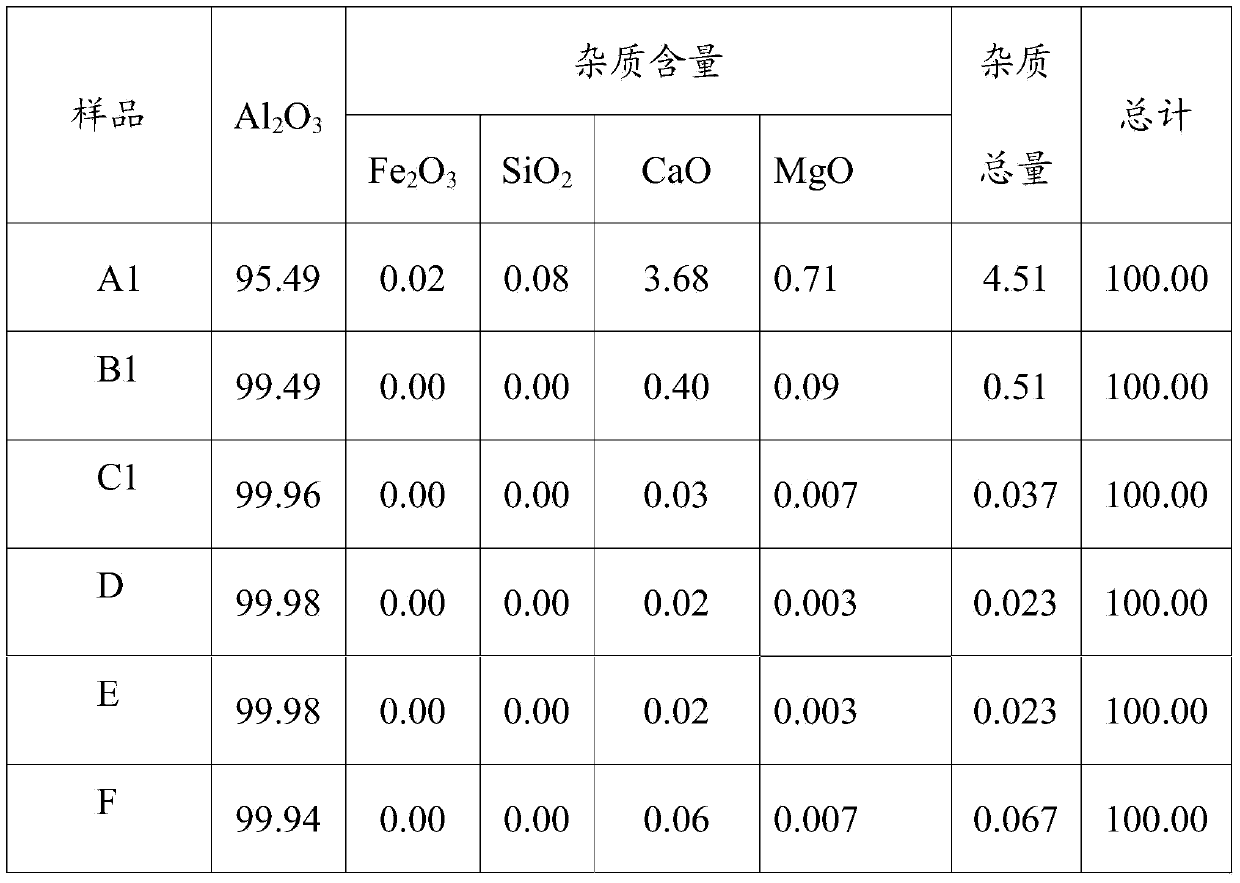
[0045] The experiment is the same as in Example 1, except that the operating temperature of the first purification in this embodiment is 50°C, the stirring time is 60min, and the purified aluminum chloride crystals are obtained after solid-liquid separation; the temperature of the second purification is 20°C , the stirring time was 60min, the secondary purified aluminum chloride crystals were obtained after solid-liquid separation, and calcined to obtain alumina D, whose main components are shown in Table 3.
Embodiment 3
[0047] The experiment is the same as in Example 2, except that the liquid-solid ratio of the first purification in this example is 6:1. The mass ratio of the concentrated hydrochloric acid added during the second purification to the crystalline aluminum chloride obtained from the first purification is 5:1. The main components of alumina E after calcination are shown in Table 3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com