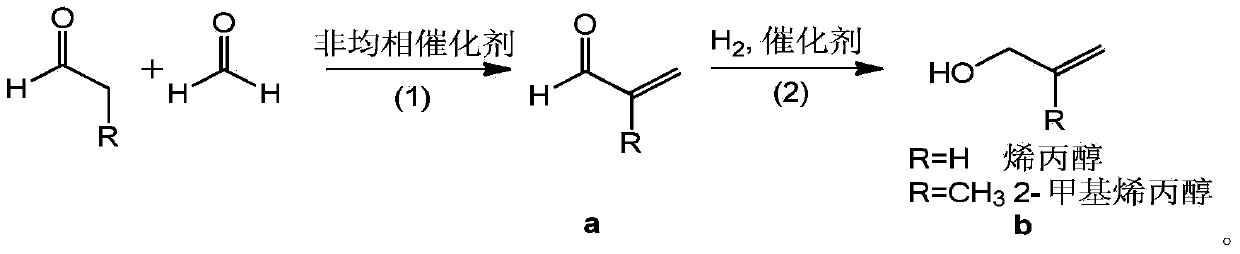
Preparation method of allyl alcohol
A technology of allyl alcohol and allyl aldehyde, which is applied in the field of preparation of allyl alcohol, can solve the problems of three wastes discharge, and achieve the effects of less by-products, high reaction selectivity, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
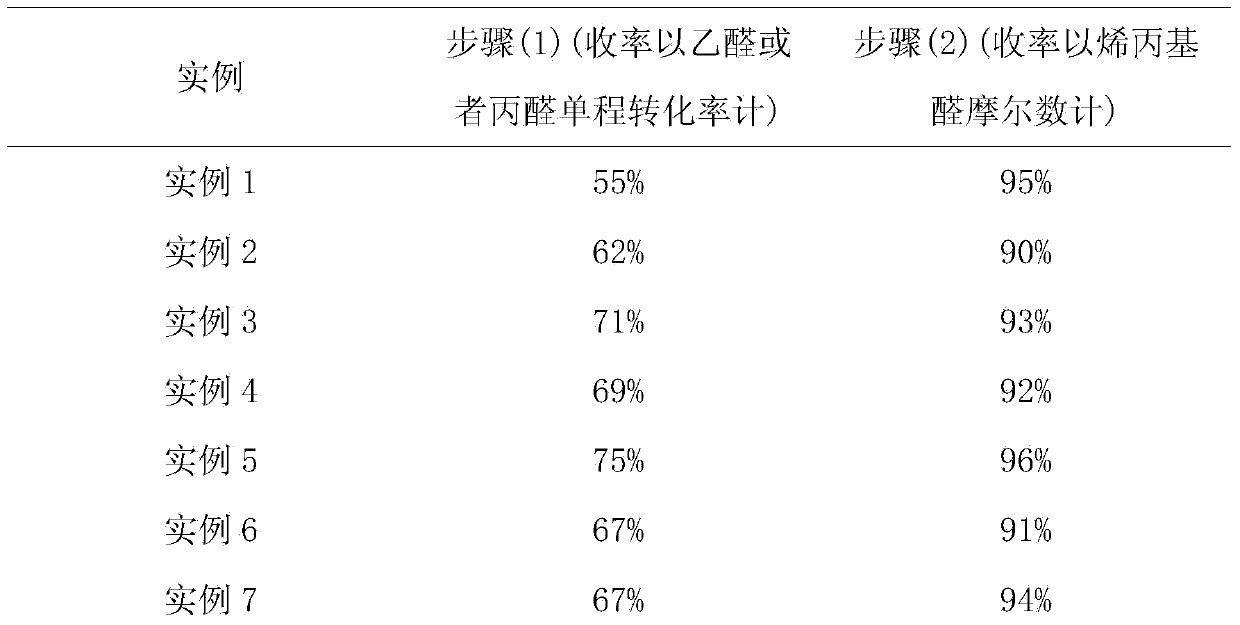
Embodiment 1
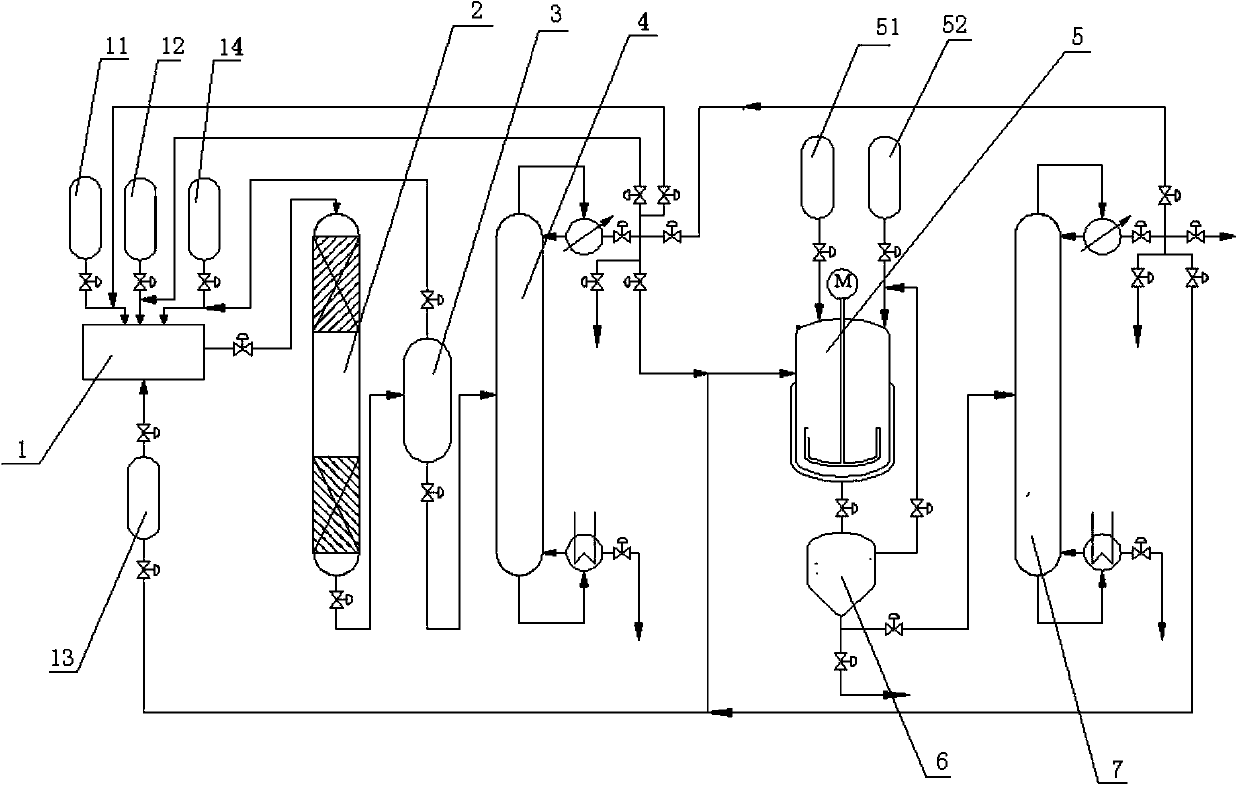
[0042] With nitrogen as the carrier gas, formaldehyde, acetaldehyde, and water are vaporized and mixed in a vaporization chamber with a temperature of 120°C, and then passed into the catalyst Li 2 O-SiO 2 In the first reaction device 2, formaldehyde, acetaldehyde, water and N 2 The molar flow ratio is controlled at 1.2:1:3:10, wherein the molar flow of acetaldehyde is controlled at 1mol / h, the temperature in the first reaction device 2 is controlled at 200°C, and the reactant is controlled in the first reaction device 2. The residence time is 30s.
[0043] After the reaction, the system is a mixture of formaldehyde, acetaldehyde, allyl aldehyde, water, carrier gas and by-products. The carrier gas is separated by cooling to 40°C-80°C through the separator 3, and then rectified by the first rectification tower 4. Separation and collection of fractions with a dew point of about 51°C-54°C to obtain allyl aldehyde, while recycling solvent water and unreacted raw materials.
[00...
Embodiment 2
[0048] With nitrogen as the carrier gas, formaldehyde, acetaldehyde, and water are vaporized and mixed in a vaporization chamber with a temperature of 180°C, and then passed into the catalyst MgO-SiO 2 In the first reaction device 2, formaldehyde, acetaldehyde, water and N 2 The molar flow ratio is controlled at 2:1:5:30, wherein the molar flow of acetaldehyde is controlled at 1mol / h, the temperature in the first reaction device 2 is controlled at 250°C, and the amount of reactant in the first reaction device 2 is controlled. The dwell time is 20s.
[0049] After the reaction, the system is a mixture of formaldehyde, acetaldehyde, allyl aldehyde, water, carrier gas and by-products. The carrier gas is separated by cooling to 40°C-80°C through the separator 3, and then rectified by the first rectification tower 4. Separation and collection of fractions with a dew point of about 51°C-54°C to obtain allyl aldehyde, while recycling solvent water and unreacted raw materials.
[00...
Embodiment 3
[0054] With nitrogen as the carrier gas, formaldehyde, acetaldehyde, and water are vaporized and mixed in a vaporization chamber with a temperature of 160°C, and then passed into the catalyst ZnO-SiO 2 In the first reaction device 2, formaldehyde, acetaldehyde, water and N 2 The molar flow ratio is controlled at 4:1:6:50, wherein the molar flow of acetaldehyde is controlled at 1mol / h, the temperature in the first reaction device 2 is controlled at 270°C, and the amount of the reactant in the first reaction device 2 is controlled. The dwell time is 15s.
[0055] After the reaction, the system is a mixture of formaldehyde, acetaldehyde, allyl aldehyde, water, carrier gas and by-products. The carrier gas is separated by cooling to 40°C-80°C through the separator 3, and then rectified by the first rectification tower 4. Separation and collection of fractions with a dew point of about 51°C-54°C to obtain allyl aldehyde, while recycling solvent water and unreacted raw materials.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com