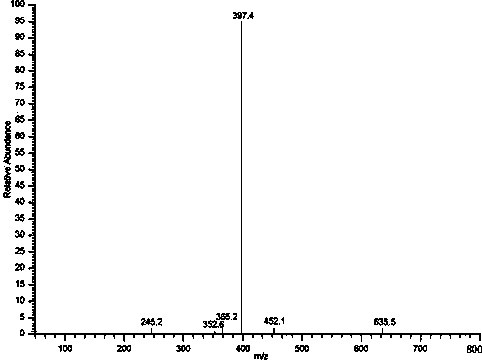
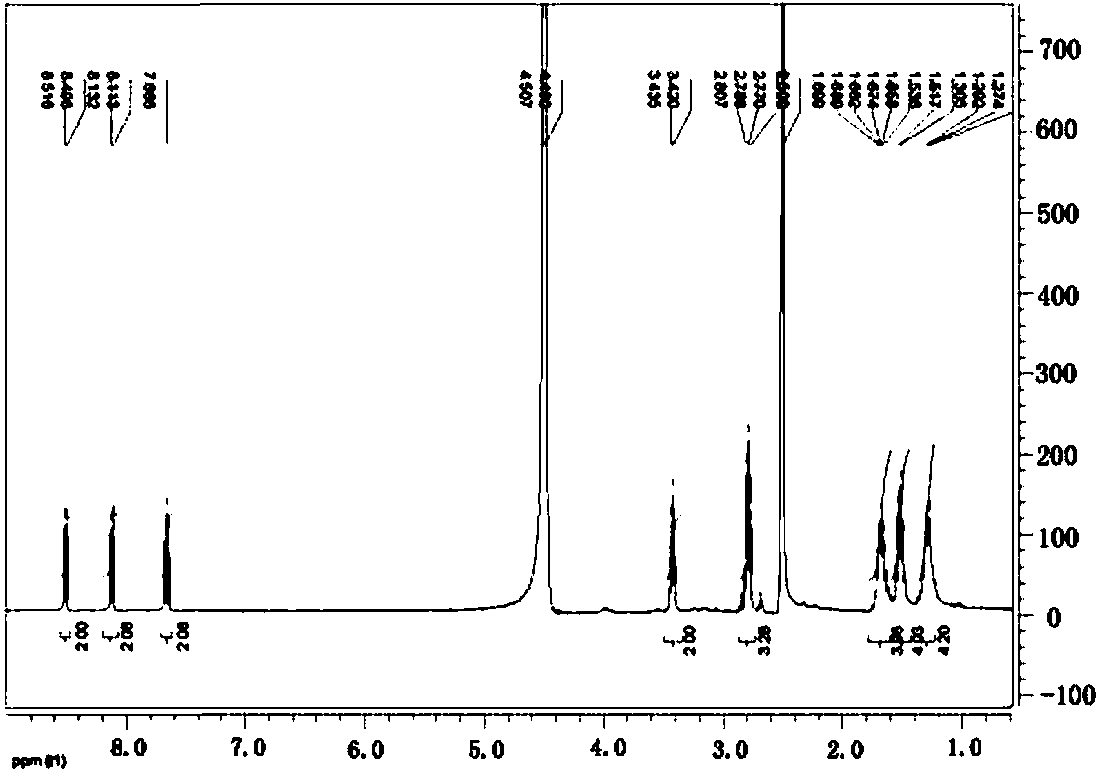
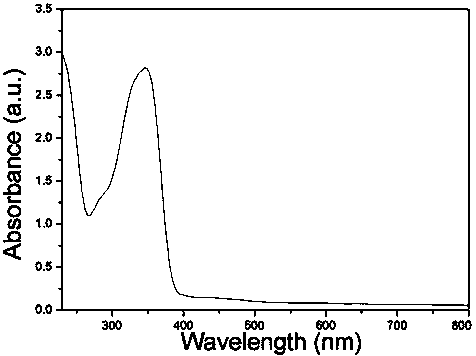
Preparation and application of naphthalimide-amino acid compound and modified quantum dot
A naphthalimide and compound technology, applied in the field of medicinal chemistry, to improve water solubility and biological properties, reduce false positive results, and the method is simple and easy to implement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052]Preparation of n = 2, x = 1, when the quantum dots (QDs) are CdSe, the synthesis steps of naphthalimide-amino acid compound and its modified quantum dots:
[0053] 1) Preparation of compound a: Add 1.97 g (10 mmol) of 1,8-naphthalimide ( ), 4.14 g (30 mmol) of anhydrous K in a 100 mL round bottom flask 2 CO 3 , 0.249 g (1.5 mmol) KI, 1.20 g (3.3 mmol) cetyltrimethylammonium bromide and 50 mL acetone, then added dropwise 6.48 g (30 mmol) 1,4-dibromobutane at 20 °C Stir the reaction for 3 days, conduct thin-layer chromatography (TCL) on a silica gel plate, discard the solid in the upper layer by suction filtration, evaporate the solvent under reduced pressure, and elute the residue with petroleum ether and ethyl acetate through flash column chromatography to obtain yellow-white crystals a;
[0054] 2) Preparation of compound b: Take lysine as an example, dissolve 1.46 g (10 mmol) lysine () in 5 mL of water, add 2 mL of saturated NaHCO 3 solution, cooled to 0°C, and slow...
Embodiment 2
[0064] Preparation of n = 2, x = 1, when the quantum dot is CdSe / CdS, the synthesis of quantum dots modified by naphthalimide-amino acid compound:
[0065] 1) Preparation of compound a: Add 1.97 g (10 mmol) of 1,8-naphthalimide ( ), 4.14 g (30 mmol) of anhydrous K in a 100 mL round bottom flask 2 CO 3 , 0.166 g (1.0 mmol) KI, 0.91 g (2.5 mmol) cetyltrimethylammonium bromide and 50 mL acetone, then 4.32 g (20 mmol) 1,4-dibromobutane was added dropwise at 30 °C Stir the reaction for 2 days, detect by thin-layer chromatography (TCL) on a silica gel plate, discard the solid in the upper layer by suction filtration, evaporate the solvent under reduced pressure, and elute the residue with petroleum ether and ethyl acetate by flash column chromatography to obtain yellow-white crystals a;
[0066] 2) Preparation of compound b: Take lysine as an example, dissolve 1.46 g (10 mmol) lysine () in 5 mL of water, add 2 mL of saturated NaHCO 3 solution, cooled to 5°C, and slowly added 1.47...
Embodiment 3
[0074] Preparation of n = 2, x = 1, when the quantum dot is CdSe / ZnS, the synthesis of quantum dots modified by naphthalimide-amino acid compound:
[0075] 1) Preparation of compound a: Add 1.97 g (10 mmol) of 1,8-naphthalimide ( ), 4.14 g (30 mmol) of anhydrous K in a 100 mL round bottom flask 2 CO 3 , 0.498 g (3 mmol) KI, 1.82 g (5 mmol) cetyltrimethylammonium bromide and 50 mL acetone, then added dropwise 10.8 g (50 mmol) 1,4-dibromobutane, stirred at 45 °C Reaction for 3 days, silica gel thin-layer chromatography (TCL) detection, suction filtration discarded the upper solid, evaporated the solvent under reduced pressure, and the residue was eluted with petroleum ether and ethyl acetate by flash column chromatography to obtain yellow-white crystals a;
[0076] 2) Preparation of compound b: Take lysine as an example, dissolve 1.46 g (10 mmol) lysine () in 5 mL of water, add 2 mL of saturated NaHCO 3 solution, cooled to -5°C, slowly added 0.733 g (3.3 mmol) of di-tert-buty...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com