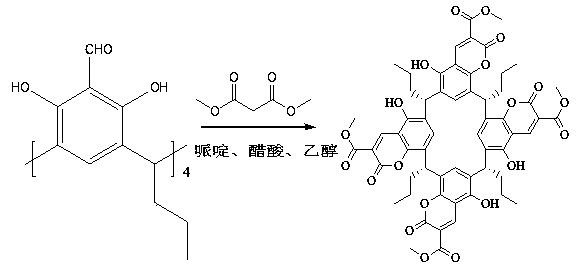
Calix (4) arene containing coumarin structure, as well as synthesis method and use thereof
A synthesis method and coumarin technology, applied in the new technology and application field of calix[4]arene synthesis, achieving the effects of high yield, mild reaction conditions and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0018] 1. Preparation process:
[0019] 1. Preparation of 2,8,14,20-tetrapropyl-5,11,17,23-tetraformyl-resorcinol calix[4]:
[0020] Add 30mL trifluoroacetic acid (TFA) in 250mL round bottom flask, add 10mmol 2,8,14,20-tetrapropyl-resorcinol calix [4] and 40mmol hexamethylenetetramine ( HMTA) mixture, heated to 85°C for 24h, then added 20mL of concentrated hydrochloric acid with a concentration of 1g / ml, and the mixture continued to react at 105°C for about 24h, and a large amount of yellow solid appeared in the system at this time. Suction filtration and recrystallization with methanol gave 2,8,14,20-tetrapropyl-5,11,17,23-tetraformyl-resorcinol calix[4] as a yellow solid with a yield of 65%.
[0021] Product identification 2,8,14,20-tetrapropyl-5,11,17,23-tetraformyl-resorcinol calix[4], yield: 65%, melting point: 294-296°C.
[0022] IR(KBr,cm -1 ): 3358(m), 2957(m), 2868(m), 1636(vs), 1463(s), 1379(m), 1241(m).;
[0023] 1 H-NMR (600MHz, CDCl 3 ):δ ppm ,0.9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com