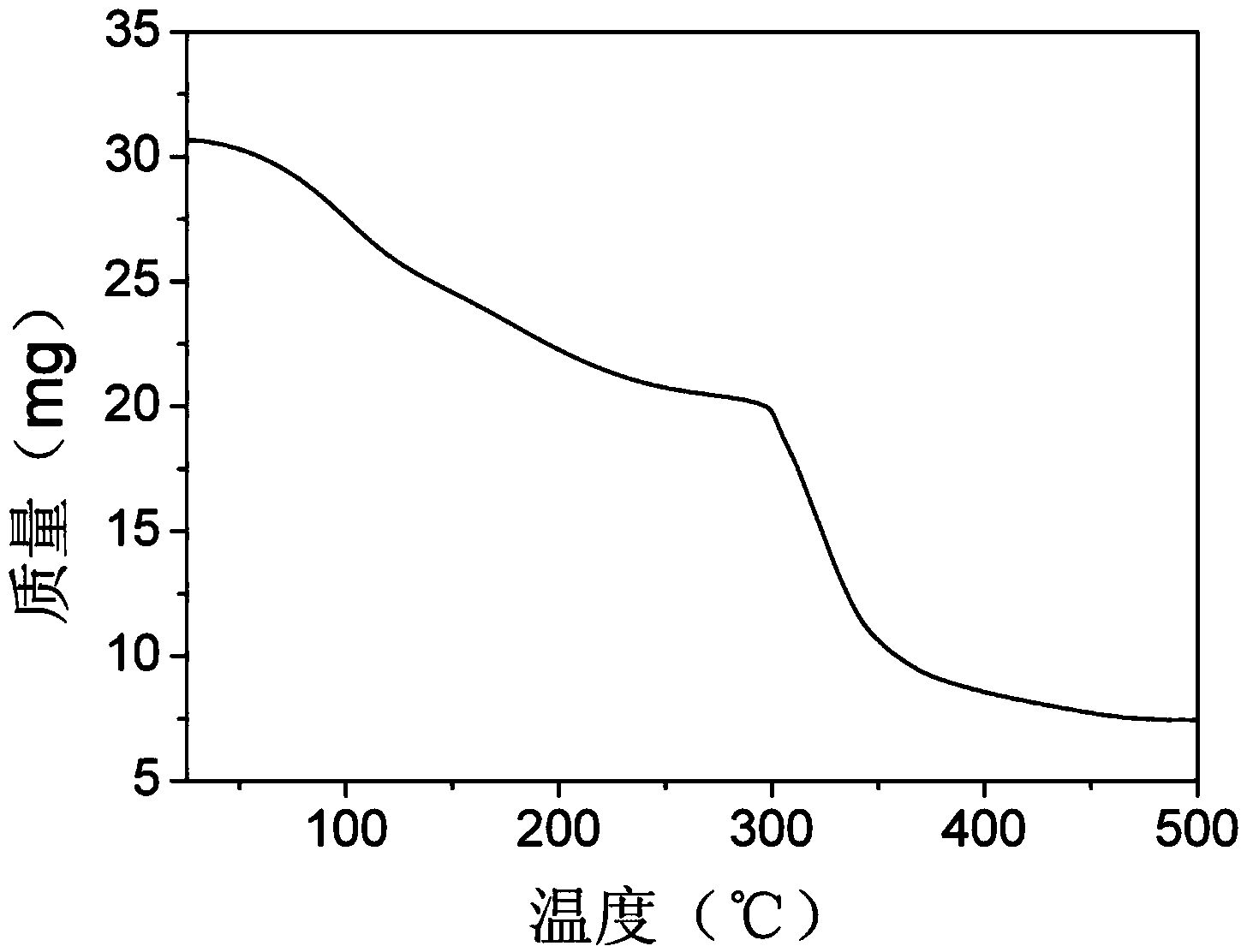
Metal-organic frame material Cu-BTC for removing nitric oxide with selective catalytic reduction method
A technology of organic framework and nitrogen oxides, applied in the field of flue gas denitrification, to achieve strong thermal stability and water resistance, increase yield, and improve synthesis conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Synthesis and Purification of Cu-BTC
[0025] Weigh 1.772g of copper nitrate trihydrate and 0.8484g of trimesic acid into 24ml of ultra-pure water and absolute ethanol respectively, and ultrasonicate the two solutions respectively. After they are all dissolved (about 5min), mix the two solutions and Transfer to a 100 ml sealable autoclave with a teflon liner. Then the reactor was placed in a preheated oven at 120°C for 24 hours. After the reaction was completed, the reactor was cooled to room temperature at normal temperature. Centrifugation was performed and the obtained samples were dried in an oven at 80°C. Purification of Cu-BTC: put 1.0g of the sample prepared in the above steps into 300ml of ultrapure water, soak it at room temperature for 8h after stirring, and centrifuge it; then, put the sample in 300ml of absolute ethanol, Soak at room temperature for 8 hours after stirring. centrifuged, washed three times with absolute ethanol, dried in an oven at 80°C af...
Embodiment 2
[0027] Activation treatment of Cu-BTC
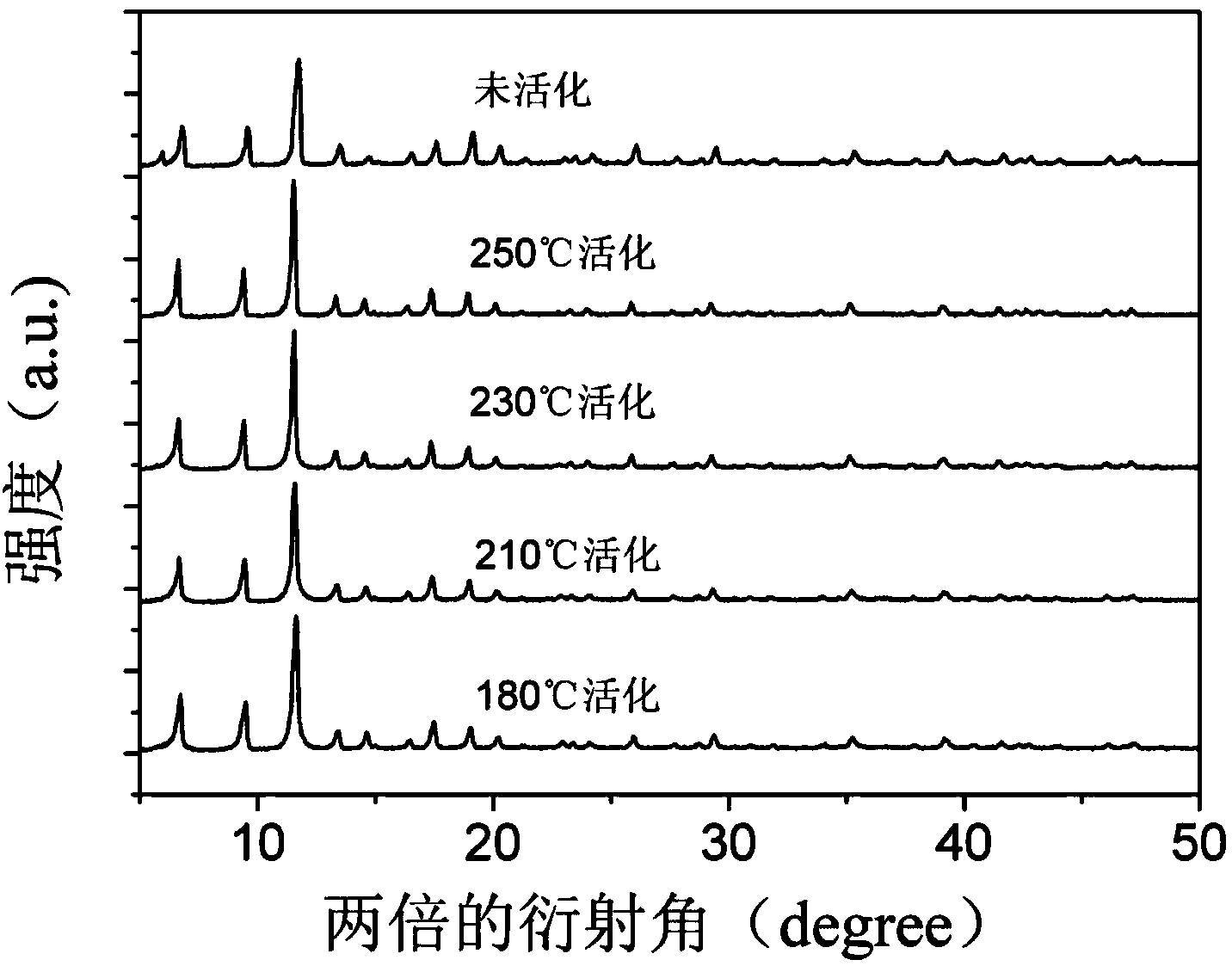
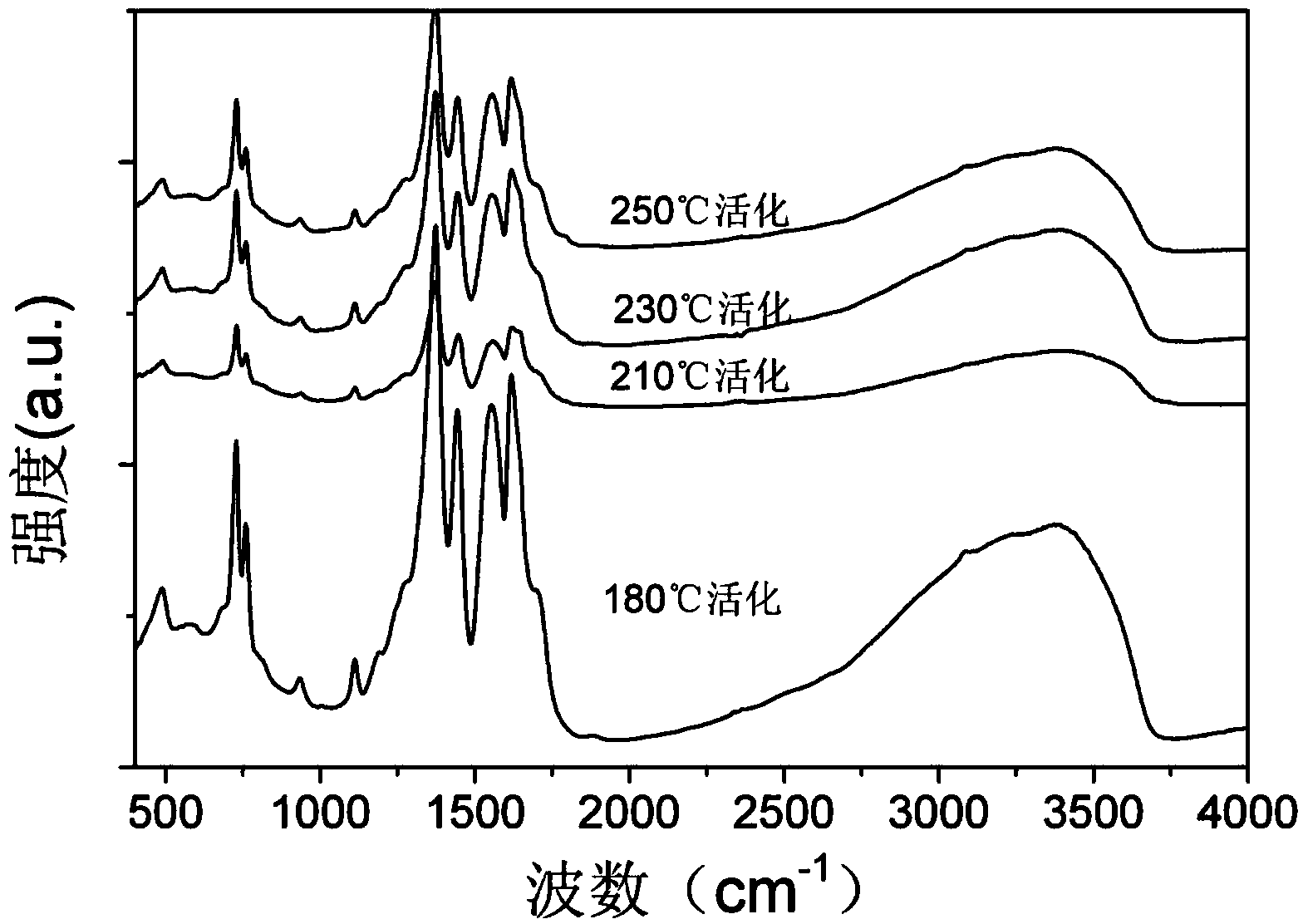
[0028] Activation of Cu-BTC: Press the purified sample into tablets, pulverize it, and sieve it to make 20-40 mesh granules. Divide the sample into 4 parts, and put about 0.3g of each part into a quartz reaction tube with an inner diameter of 6mm. Continuously feed high-purity N at 30ml / min 2 , heated at a rate of 10°C / min from room temperature to different temperatures for activation, the temperatures were 180°C, 210°C, 230°C, 250°C and then kept at this temperature for 3h. XRD patterns of different catalysts, such as figure 2 . Among them, the unactivated Cu-BTC has one more peak at about 6° than the activated Cu-BTC, and there is no other difference. This peak may be due to dehydration after activation. There was no difference between the catalysts activated at each temperature. Infrared characterization diagrams of each catalyst, image 3 . The different peak intensities of the various catalysts are caused by the different am...
Embodiment 3
[0030] Activity test of Cu-BTC activated at 180°C
[0031] The specific experimental process is as follows: 0.2 g of the Cu-BTC catalyst activated at 180° C. prepared in Example 2 was weighed and loaded into a fixed-bed reactor with an inner diameter of 6 mm and a heating function. Simulated flue gas conditions: 500ppm NO, 500ppm NH 3 , 5%O 2 , balance gas N 2 , the flow rate is 100ml / min, and the temperature range is 140~300℃. The simulated flue gas passes through the fixed-bed reactor equipped with catalyst, and is heated. Points are taken within the above temperature range and the NO concentration value is recorded by the flue gas analyzer. It was measured that the NO conversion rate was 45% at 200°C and reached 100% at 240°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com