Preparation method and application of a surface-mounted linear silver photocatalyst
A catalyst and assembly line technology, applied in chemical instruments and methods, physical/chemical process catalysts, chemical/physical processes, etc., to achieve high catalytic activity, accelerated oxidation decomposition rate, and simple and easy preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] At room temperature, take 30mL of water, pour it into a beaker, add 0.05g of copper micron wire (the molar number of copper element is 0.7874mmol) under stirring conditions, then add 0.1575g (1.092mmol) of urotropine, 0.8g (0.016mmol) polyvinylpyrrolidone (K-30, Sinopharm Chemical Reagent Co., Ltd.), and ultrasonically oscillated to uniformly disperse elemental copper in the system to obtain a copper dispersion.
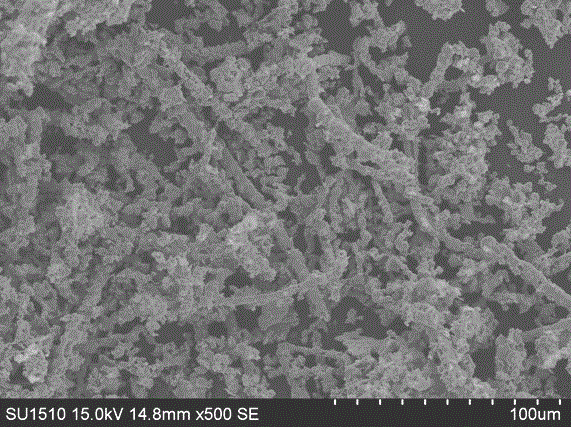
[0020] Pour 10ml of silver nitrate aqueous solution (0.1872mol / L) with a concentration of 3.08% by mass into the copper dispersion at one time to carry out a displacement reaction at a temperature of 10°C. When the solution turned black gray, the reaction was completed, and the precipitate was collected by centrifugation, washed, dried at 50°C for 5h, and then calcined at 450°C for 2h to obtain material 1. X-ray electron diffraction image from material 1 ( Figure 4 ) It can be seen that material 1 is pure phase silver. From the electron microscopy (SEM) top...
Embodiment 2
[0022] At room temperature, take 30mL of water, pour it into a beaker, add 0.0396g of copper micron wire (the molar number of copper is 0.6236mmol) under stirring conditions, then add 0.1731g (0.0012mol) of urotropine, 0.9g (0.018mmol) polyvinylpyrrolidone (K-30, Sinopharm Chemical Reagent Co., Ltd.), and ultrasonically oscillated to disperse elemental copper in the system uniformly to obtain a copper dispersion.
[0023] Pour 10ml of silver nitrate solution (0.1872mol / L) with a concentration of 3.08% by mass into the copper dispersion at one time to carry out the displacement reaction, and the reaction temperature is 15°C. When the solution turned black gray, the reaction was completed, and the precipitate was collected by centrifugation, washed, dried at 70°C for 1 hour, and then calcined at 500°C for 1 hour to obtain material 2. From the X-ray electron diffraction image (XRD) of material 2, it can be seen that material 2 is pure phase silver. From the electron microscope (...
Embodiment 3
[0025] At room temperature, take 30mL of water, pour it into a beaker, add 0.1189g of copper micron wire (the molar number of copper is 1.87mmol) under stirring conditions, then add 0.0865g (0.5998mmol) of urotropine, 0.6g (0.012mmol) polyvinylpyrrolidone (K-30), and ultrasonically oscillate to disperse the elemental copper in the system uniformly to obtain a copper dispersion.
[0026] Pour 10ml of silver nitrate solution (0.1872mol / L) with a concentration of 3.08% by mass into the copper dispersion at one time to carry out a displacement reaction at a temperature of 20°C. When the solution turned black gray, the reaction was completed, and the precipitate was collected by centrifugation, washed, dried at 60°C for 3 hours, and roasted at 400°C for 3 hours to obtain material 3. From the X-ray electron diffraction image (XRD) of material 3, it can be seen that material 3 is pure phase silver. From the electron microscope (SEM) topography of material 3, it can be seen that line...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com