Methods for preparing ethambutol and ethambutol hydrochloride
A technology of ethambutol hydrochloride and ethambutol, which is applied in chemical instruments and methods, preparation of organic compounds, preparation of aminohydroxy compounds, etc., can solve the problem of restricting the production progress of large-scale production, rolling feed production, occupying a long time for equipment, and being difficult to Controlling the feeding amount and other issues to achieve the effect of improving solvent recovery rate, reducing corrosion to equipment and high practical value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
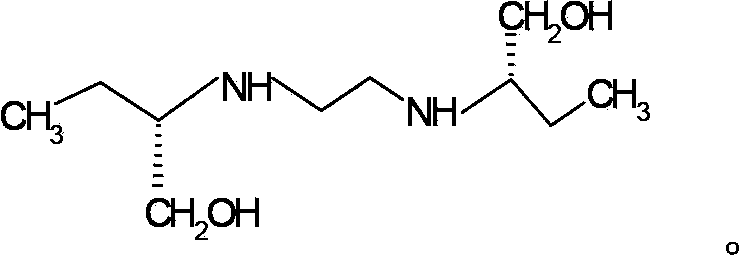
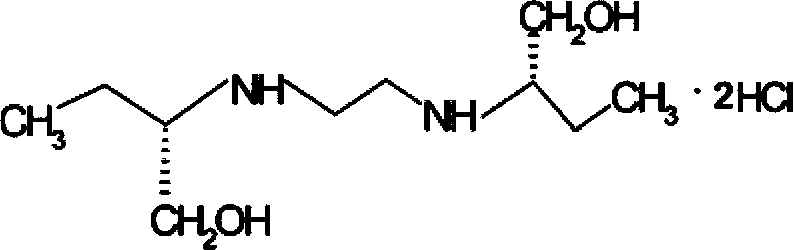
[0051] The method for preparing ethambutol hydrochloride of the present invention comprises utilizing (S)-2-aminobutanol and 1,2-dichloroethane to carry out condensation reaction to prepare ethambutol, wherein said condensation reaction is in a low-boiling point organic solvent carried out, and the HCl generated during the reaction was neutralized with ammonia gas.
[0052] The method may further include:
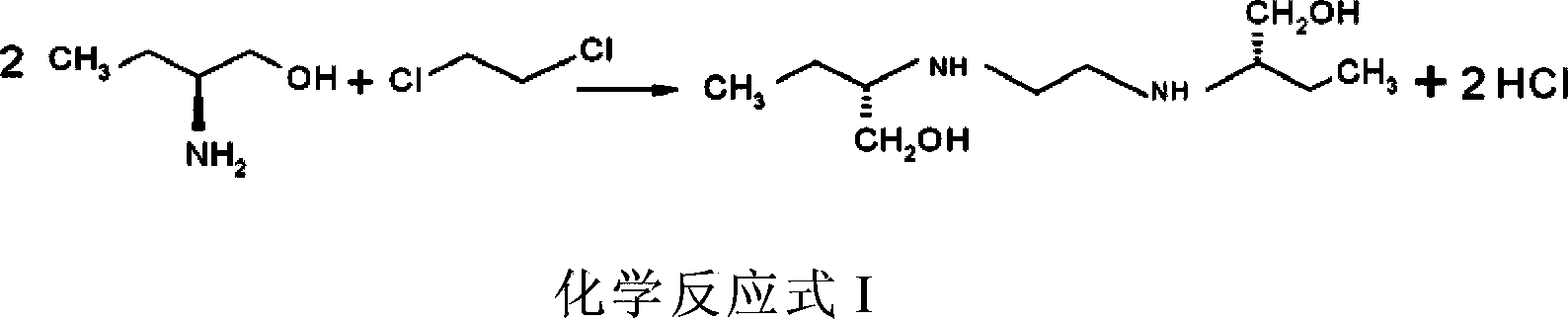
[0053] 1) Make excess (S)-2-aminobutanol and 1,2-dichloroethane undergo condensation reaction as shown in chemical reaction formula I to generate ethambutol and HCl:
[0054]
[0055] Wherein, the condensation reaction is carried out in a low-boiling point organic solvent, and the reaction temperature is 70-80°C;
[0056] 2) Neutralize the generated HCl with ammonia gas, and make the pH of the resulting reaction system 9-10;
[0057] 3) Separating the reaction system obtained in step 2) to obtain ethambutol.
[0058] (S)-2-aminobutanol and 1,2-dichloroethane are known...
Embodiment 1
[0101] Put 735.3ml (765g, 8.58mol) of (S)-2-aminobutanol and 114ml (90g) of absolute ethanol into a 1.5L reaction tank, stir to raise the temperature, control the temperature to 78°C, and slowly add 71.4 Add ml (90g, 1mol) of 1,2-dichloroethane within 2.5 hours, control the temperature at 80°C, and keep it warm for another 7 hours. The temperature was lowered to 58°C, and 42.6 g (2.5 mol) of ammonia gas was introduced slowly, and the addition was completed within 2.5 hours. Control the pH of the reaction solution to 9.7. The temperature was raised to 95°C to distill and recover 107ml (84.5g) of ethanol. Use vacuum distillation to recover 586.7ml (563.3g, 6.32mol) of (S)-2-aminobutanol at 153°C, and control the vacuum pressure to -0.09MPa, leaving 274g of the product in the tank. Cool down to 70°C, add 300ml (237g) of absolute ethanol, stir for 0.5 hours, filter with suction, filter off ammonium chloride (84.9g, 1.6mol), and obtain 425g (319ml) of ethambutol alcohol solution,...
Embodiment 2
[0103] Put 735.3ml (765g, 8.58mol) of (S)-2-aminobutanol and 114ml (90g) of anhydrous methanol into a 1L reaction tank, stir to raise the temperature, control the temperature to 78°C, and slowly add 90g ( 1mol) of 1,2-dichloroethane was added within 2.5 hours, and then kept for 7 hours. The temperature was lowered to 55°C, and 42.6g (2.5mol) of ammonia gas was introduced slowly, and the addition was completed dropwise within 2.5 hours, and the pH of the reaction solution was controlled to be 9.5. The temperature was raised to 95°C to recover 107.5ml (85g) of anhydrous methanol. 578.4ml (555.3g, 6.23mol) of (S)-2-aminobutanol was recovered by vacuum distillation at 154°C, the vacuum pressure was controlled at -0.09MPa, and 262g of the product remained in the tank. Cool down to 70°C, add 300ml (237g) of absolute ethanol, stir for 0.5 hours, filter with suction, filter off 83g (1.55mol) of ammonium chloride, obtain 416g (318ml) of ethambutol alcohol solution, and recover ethanol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com