Rhodococcus erythropolis XS1012 and application thereof in preparation of chiral alcohol
A technology of XS1012, Rhodococcus erythraea, applied in the direction of bacteria, microorganism-based methods, biochemical equipment and methods, etc., can solve the problem of limited industrial application, complex separation and purification steps of aldehyde-ketone reductase or alcohol dehydrogenase, and cost of coenzymes. expensive, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Embodiment 1: the acquisition of wet thalline
[0055] Fermentation medium formula: glucose 15g / L, peptone 7.5g / L, yeast extract 6g / L, (NH 4 ) 2 SO 4 3g / L, KH 2 PO 4 1.5g / L, NaCl0.75g / L, MgSO 4 ·7H 2 O0.75g / L, the solvent is water, pH6.5.
[0056] The formula of the seed medium is the same as that of the fermentation medium.
[0057] 1) Slant culture: Pick a single colony of Rhodococcus erythropolis XS1012 and inoculate it into the slant medium, culture at 30°C for 2-3 days, and store in a refrigerator at 4°C; the final concentration of the slant medium is composed of: glucose 15g / L, peptone 7.5g / L , yeast extract 6g / L, (NH 4 ) 2 SO 4 3g / L, KH 2 PO 4 1.5g / L, NaCl0.75g / L, MgSO 4 ·7H 2 O0.75g / L, agar 20g / L, solvent is water, pH6.5.
[0058] 2) Seed culture: Pick a ring of bacteria from the mature slant and insert it into the seed medium, cultivate at 30°C and 200rpm for 24 hours, and obtain the seed liquid;
[0059] 3) Fermentation culture: Transfer the se...
Embodiment 2~4
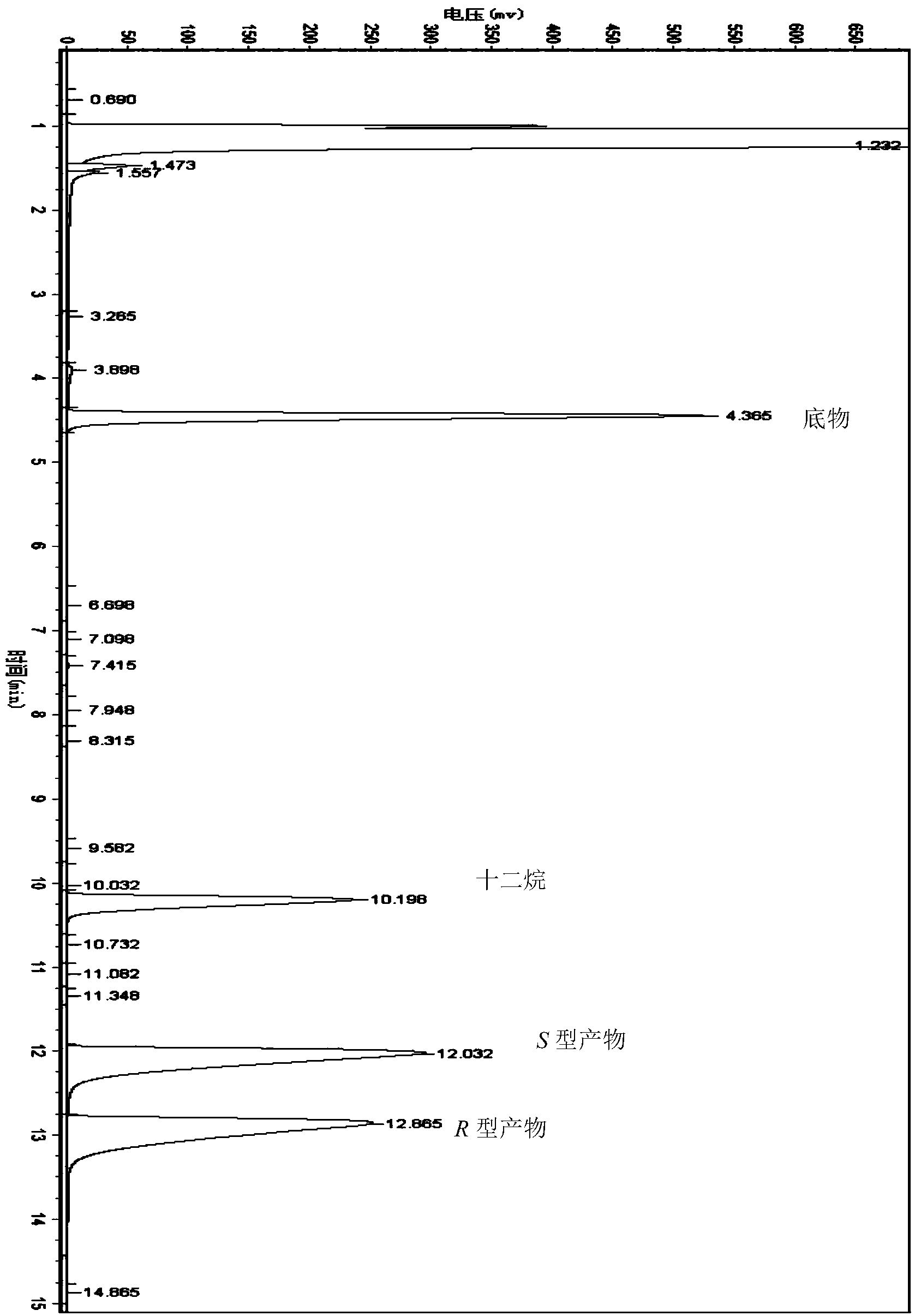
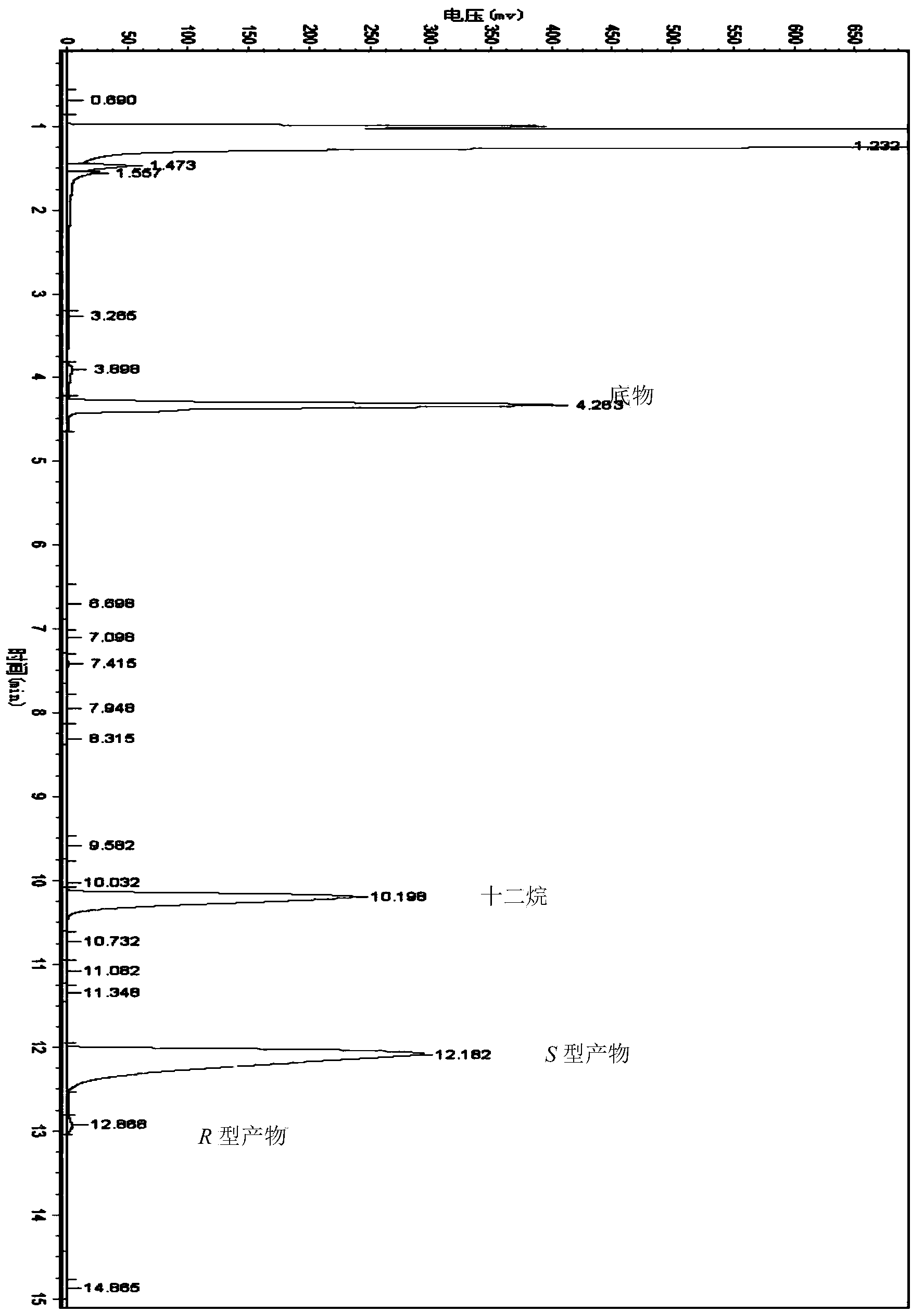
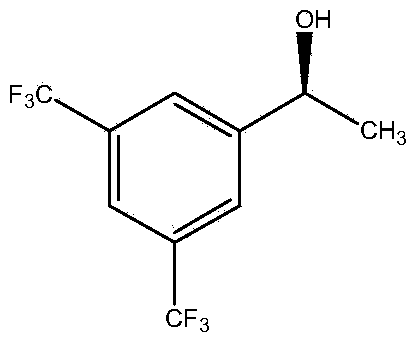
[0061] The wet thalline of embodiment 1 gained is suspended in the phosphate buffer saline (Na 2 HPO 4 -KH 2 PO 4 buffer), the concentration of the wet bacteria is 35g / L based on the dry weight of the bacteria; add [3,5-bis(trifluoromethyl)phenyl]ethanone with a final concentration of 20mmol / L as a substrate, and then add Glucose with a final concentration of 50g / L was used as an auxiliary substrate, and placed in a shaking table at 30°C and 200rpm for 24 hours. After the reaction, the reaction liquid was centrifuged, and the supernatant was extracted by adding an equal volume of n-hexane. Gas chromatographic analysis, quantitative with internal standard method. The internal standard is dodecane. Take 1ml of the extract and add 2μl of dodecane for analysis. Gas chromatography conditions: Japan Shimadzu GC-2014 gas chromatograph, Zhejiang University N2000 chromatography workstation; American Varian CP-Chirasil-Dex chiral capillary gas chromatography column (25m×0.25mm×0.25...
Embodiment 5~10
[0065] The wet thalline obtained in Example 1 was suspended in 10 mL of different phosphate buffer systems (pH 7.0), and the wet thalline was 35 g / L in terms of dry weight of the thalline; adding [3, 5-bis(trifluoromethyl)phenyl]ethanone is used as substrate, then glucose with a final concentration of 50g / L is added as auxiliary substrate, placed at 30°C, and reacted in a shaker at 200rpm for 24h. After the reaction, the The reaction solution was centrifuged, and the supernatant was added to an equal volume of n-hexane for extraction. The extract was analyzed by gas chromatography and quantified by the internal standard method. The concentration and ee value of the product (S)-[3,5-bis(trifluoromethyl)phenyl]ethanol are shown in Table 2. As can be seen from Table 2, when the buffer system is Na 2 HPO 4 -KH 2 PO 4 , the concentration of (S)-[3,5-bis(trifluoromethyl)phenyl]ethanol was the highest at 14.6mmol / L, the ee value was 99.9%, and the yield was 73.0%.
[0066] The c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com