Process for synthesizing diisopropylamine from isopropylamine
A technology for diisopropylamine and isopropylamine, applied in the field of synthesizing diisopropylamine, can solve the problems of shortcoming in catalyst stability, short process flow, short service life, etc., and achieve the effects of reduced scale, fewer regeneration times, and reduced production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
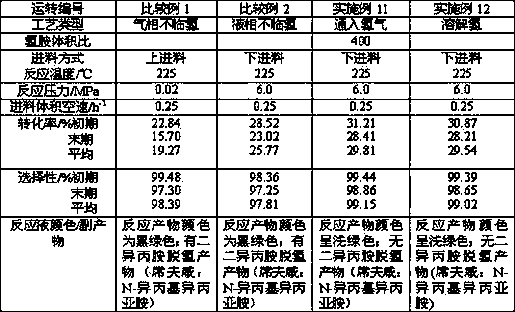
[0028] 70g of Hβ zeolite (SiO 2 / Al 2 o 3 The molecular ratio is 30) into 250mL 0.2M KCl solution, ion exchange is carried out at a temperature of 90~95°C, and the exchange time is 2.0h; after that, filter and wash until there is no Cl - ; Dry at 60°C for 4.0h, 110°C for 4.0h; then mix with 10g of aluminum hydroxide powder, add nitric acid and deionized water, knead and extrude on the extruder, and the amount of nitric acid added accounts for 0.5% of the dry material w%, the amount of deionized water added is subject to kneading and extruding. The strip-shaped catalyst was dried at 110°C for 4.0h, and then calcined in a muffle furnace at 550°C for 4.0h to obtain K / Hβ zeolite-Al 2 o 3 Catalyst, which contains 0.9w% potassium, 92.8w% Hβ zeolite, and the balance is γ-Al 2 o 3 , the catalyst number is AF-1.
Embodiment 2
[0030] Take 70g of Hβ zeolite (SiO 2 / Al 2 o 3 The molecular ratio is 42), according to the method described in Example 1, K / Hβ zeolite-Al 2 o 3 Catalyst, which contains potassium 1.6w%, contains Hβ zeolite 85.8w%, and the balance is γ-Al 2 o 3 , the catalyst number is AF-2.
Embodiment 3
[0032] Take 70g of Hβ zeolite (SiO 2 / Al 2 o 3 Molecular ratio is 66), according to the method described in embodiment 1, K / Hβ zeolite-Al is prepared 2 o 3 Catalyst, which contains potassium 1.6w%, contains Hβ zeolite 85.8w%, and the balance is γ-Al 2 o 3 , the catalyst number is AF-3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com