Compound and application of compound in preparation of drugs for resisting parasitic diseases
A compound and reaction technology, applied in the field of application in the preparation of antiparasitic drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] Embodiment 1, the preparation of compound
[0091] 1. Intermediate A 1 preparation of
[0092] For the preparation flow chart, see figure 1 .
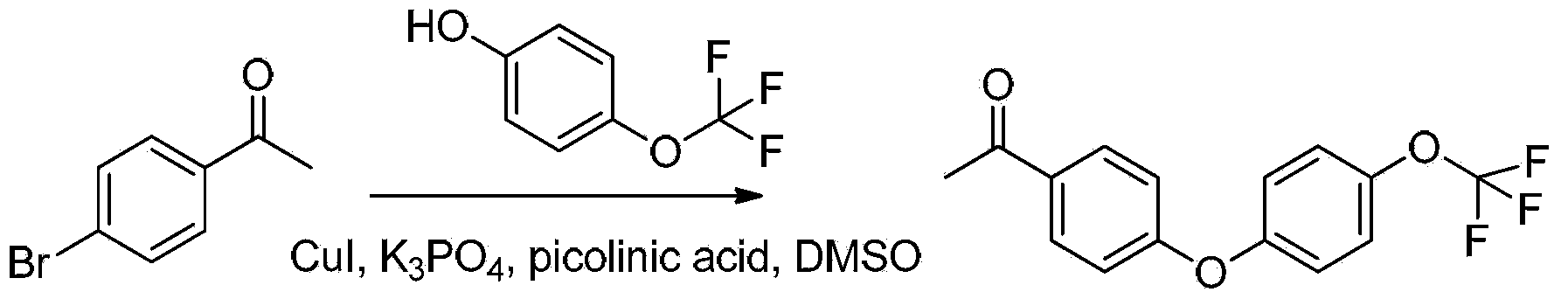
[0093] Add p-3g p-bromoacetophenone, 570mg cuprous iodide, 738mg 2-pyridinecarboxylic acid, 6.4g potassium phosphate, 3.9ml p-trifluoromethoxyphenol and 15ml dimethyl sulfoxide in a round bottom flask, under argon Heating and stirring at 110°C for 20 hours under gas-protected conditions, then cooling to room temperature, adding 100ml of water to dilute, then extracting with dichloromethane 3 times (adding 25ml of dichloromethane each time), combining the organic phases extracted three times, washing with water After two times, dry with anhydrous sodium sulfate, then spin dry the solvent under reduced pressure, and pass the residue through a silica gel column with petroleum ether: ethyl acetate = 25:1 (volume ratio) to obtain 3.7g of intermediate A 1 (Intermediate A 1 As light yellow liquid), the yield was 84%.
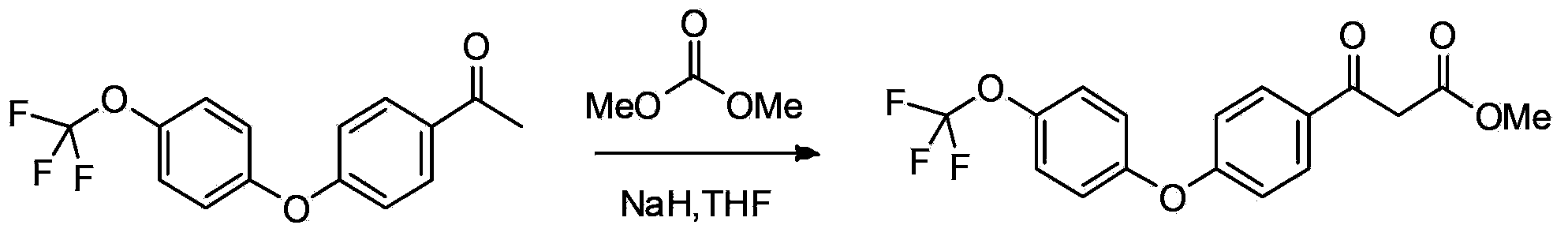
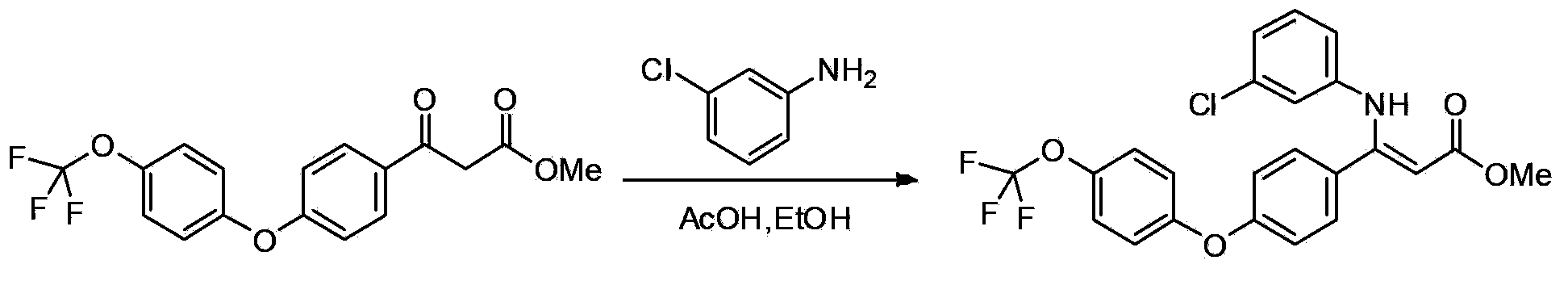
[0094] 2. ...
Embodiment 2
[0163] Example 2, the inhibitory effect of the compound on the activity of NDH-2 protein
[0164] 1. Experimental principle
[0165] The chemical reaction catalyzed by NDH-2 (type II NADH dehydrogenase) is: NADH+UQ→NAD + +UQH 2 ; Among them, NADH has light absorption at 340nm, so the reduction rate of NADH can be evaluated by the decrease of light absorption value at 340nm.
[0166] 2. Preparation of experimental materials
[0167] (1) Preparation of NDH-2 protein
[0168] Express the NDH-2 protein of Plasmodium falciparum 3D7 strain in Escherichia coli (the NDH-2 protein is made up of 533 amino acid residues, as shown in sequence 1 of the sequence table, the 1st to 24th amino acid residues from the N-terminal are signals The peptide is used to locate the protein on the inner mitochondrial membrane of Plasmodium; the NDH-2 gene consists of 1602 nucleotides, as shown in sequence 2 of the sequence table), and the specific steps are as follows:
[0169] 1. Synthesize the dou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Absorption coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com