Mutant endoglucanase?
An endoglucanase and mutant technology, applied in the direction of glucose production, enzymes, hydrolytic enzymes, etc., can solve the problems of unknown inhibition mechanism, achieve good efficiency, high-efficiency decomposition, and reduced activity inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
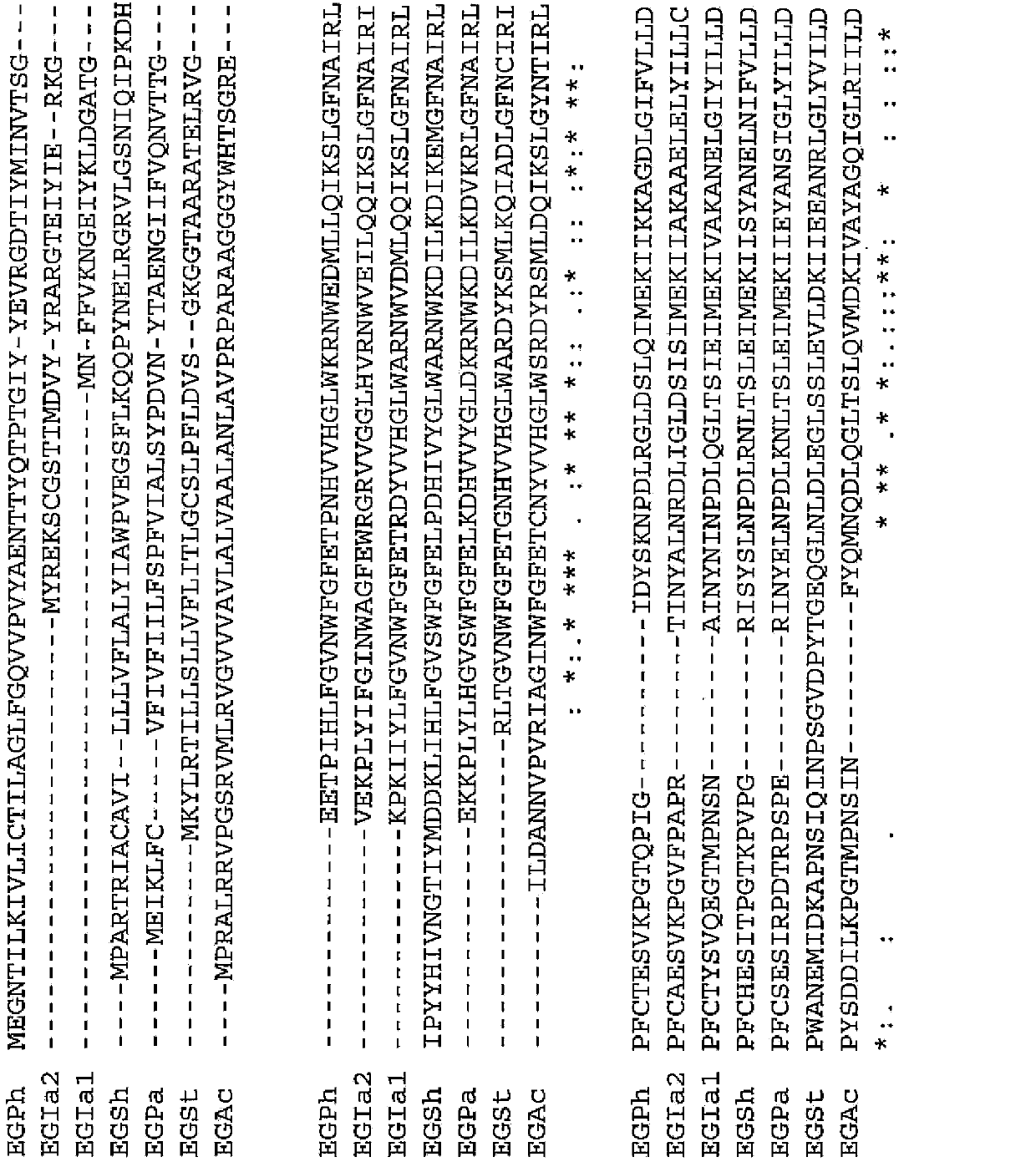
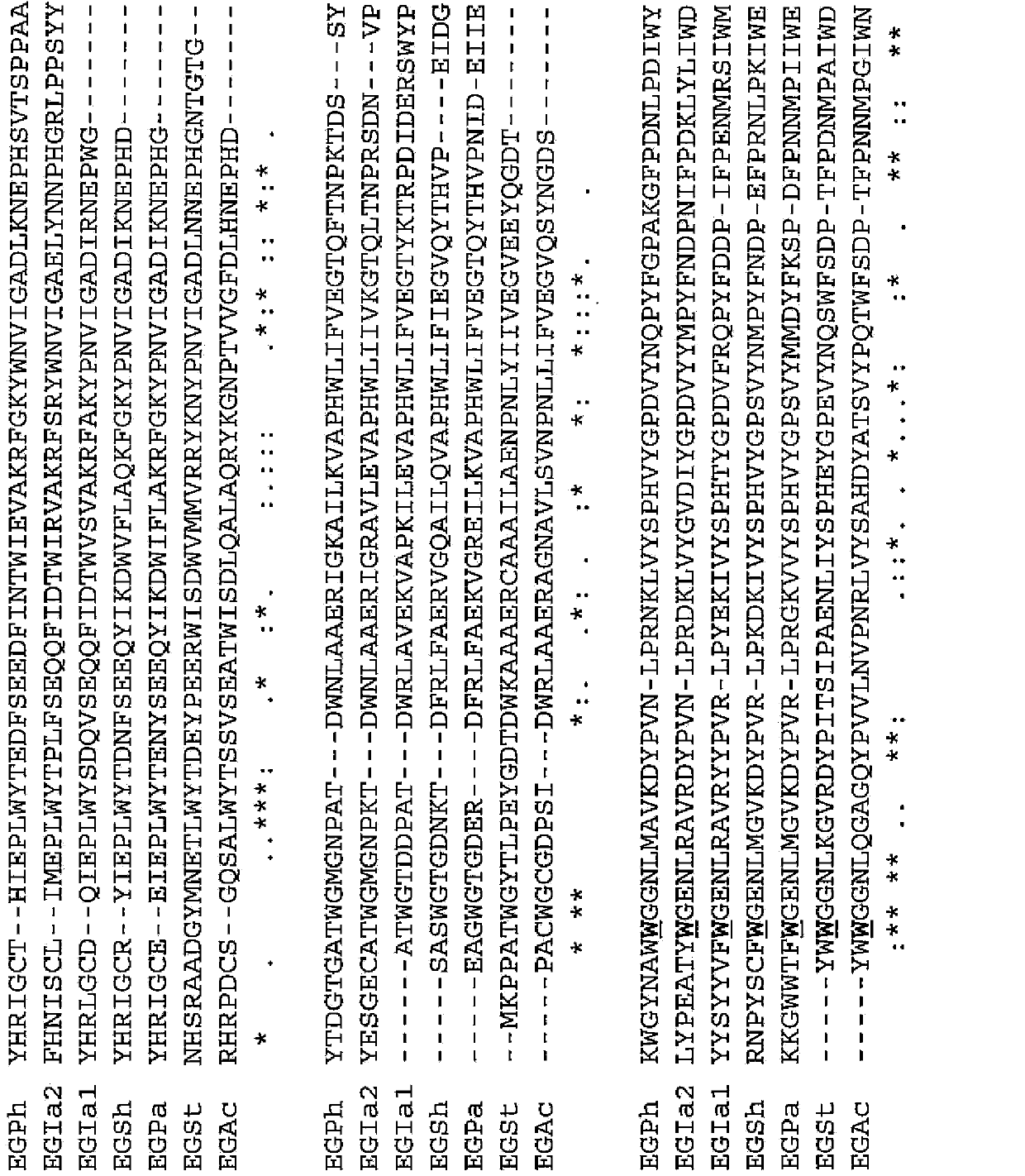
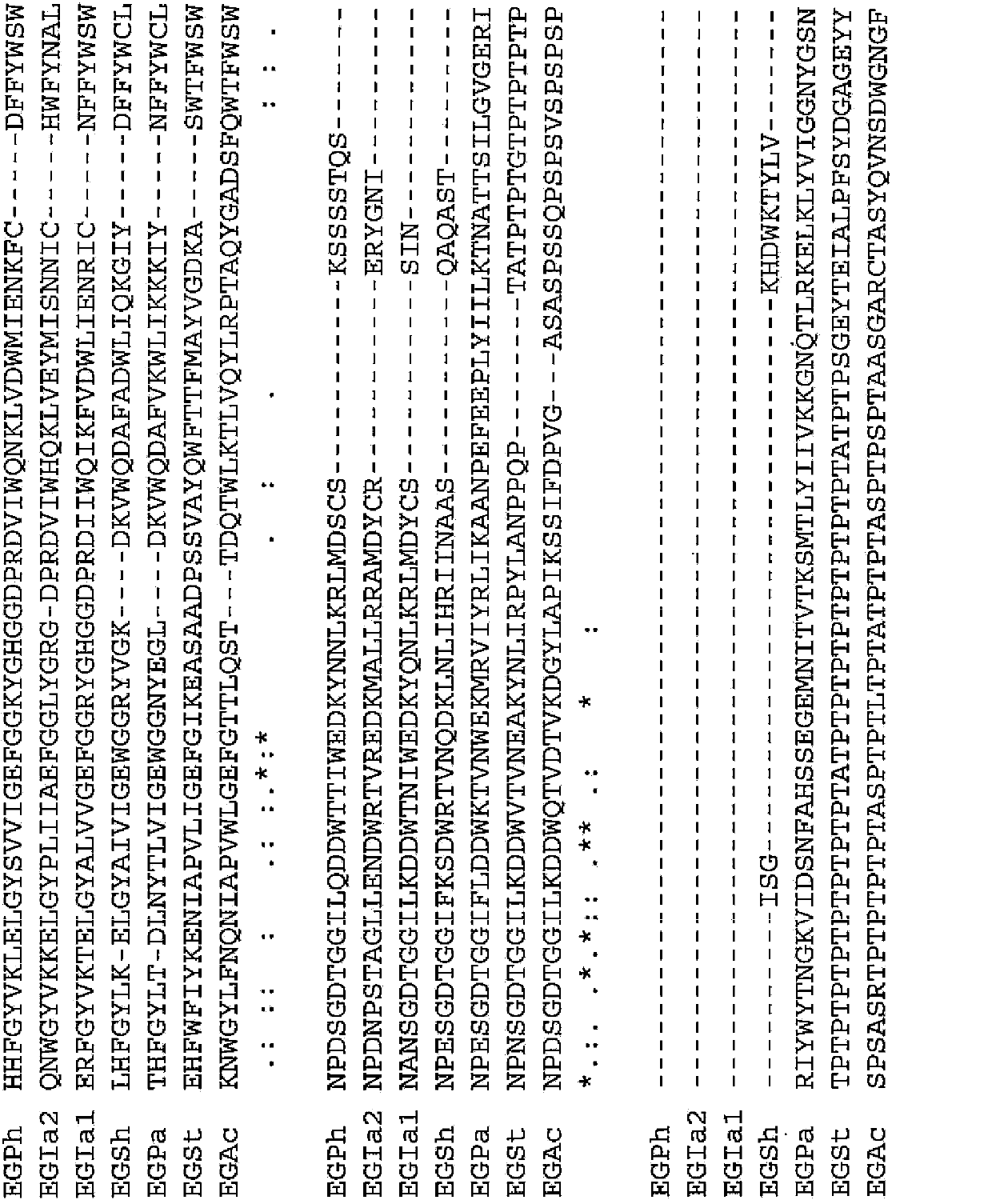
[0091] (Example 1) Determination of the 273rd amino acid residue in the endoglucanase derived from thermophilic bacteria
[0092] A BLAST search was performed to search for endoglucanases derived from thermophilic bacteria having high amino acid sequence identity with EGPh.
[0093] For the BLAST search, ProteinBLAST was used using sequence number 1 as a query. As a result, endoglucanase 1 (EGIa1) derived from Ignisphaera aggregans described in SEQ ID NO: 7 was found as an endoglucanase derived from thermophilic bacteria showing 75% or more identity with EGPh, sequence Endoglucanase 2 (EGIa2) derived from Ignisphaera aggregans described in No. 13, endoglucanase (EGSh) derived from Staphylothermus hellenicus described in SEQ ID No. 19, derived from Pyrococcus abyssi described in SEQ ID NO: 25 Endoglucanase (EGPa), the endoglucanase (EGAc) derived from Acidthermus cellulolyticus described in SEQ ID NO: 31, the endoglucanase (EGAc) derived from Sequence No. Thermophila) endoglu...
reference example 1
[0095] (Reference Example 1) Preparation of pro-endoglucanase
[0096] EPGh, EGIa1, EGIa2, EGSh, EGPa, EGAc, and EGSt genes were completely synthesized from the genes described in SEQ ID NOs: 1, 7, 13, 19, 25, 31, and 37, respectively, using DNA Ligation Kit (タカラバイオ) and NcoI and BamHI of pET11d were ligated to transform JM109 (タカラバイオ). Selection was performed using LB agar medium containing ampicillin as an antibiotic. The prepared vectors pET-EGPh, EGIa1, EGIa2, EGSh, EGPa, EGAc, and EGSt were isolated from the transformed JM109 strain using a mini-extraction kit (Kiagen) and subjected to base sequence analysis. Escherichia coli BL21(DE3) pLysS strain for expression was transformed with pET-EGPh, EGIa1, EGIa2, EGSh, EGPa, EGAc, and EGSt to prepare BL21-PfuBGL strain. The BL21-PfuBGL strain was inoculated in 10 mL of LB medium containing ampicillin, and cultured with shaking at 37° C. overnight (preculture). As main culture, the bacterial cells obtained before inoculation...
Embodiment 2
[0097] (Example 2) Preparation of mutant endoglucanase
[0098] The mutant endoglucanase of the present invention was produced by the following method using the primer pairs shown in Table 1.
[0099] Table 1
[0100]
[0101] For the gene encoding the amino acid sequence shown in SEQ ID NO: 1, mutant EGPh (SEQ ID NO: 2) was produced by site-directed mutagenesis using oligonucleotides shown in the base sequences of SEQ ID NOs: 5 and 6. In addition, similarly, for the gene encoding the amino acid sequence shown in SEQ ID NO: 7, mutant EgIa1 (SEQ ID NO: 8) was produced using the oligonucleotides shown in the base sequences of SEQ ID NO: 11 and 12, and for the gene encoding the amino acid sequence shown in SEQ ID NO: 13 For the gene of the amino acid sequence shown in SEQ ID NO: 17 and 18, mutant EgIa2 (SEQ ID NO: 14) was made, and for the gene encoding the amino acid sequence shown in SEQ ID NO: 19, mutant EGSh (SEQ ID NO: 20) was made using SEQ ID NO: 23 and 24 ), for the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com