Pharmaceutical composition for treating hypertension and preparation method thereof
A pharmacy and reserpine technology, applied in the direction of drug combination, pharmaceutical formula, heterocyclic compound active ingredients, etc., can solve the problems affecting the quality of medicines, the instability of dihydralazine sulfate, the instability of dihydralazine sulfate and vitamin B1 and other problems, to achieve the effect of stable components and simple preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
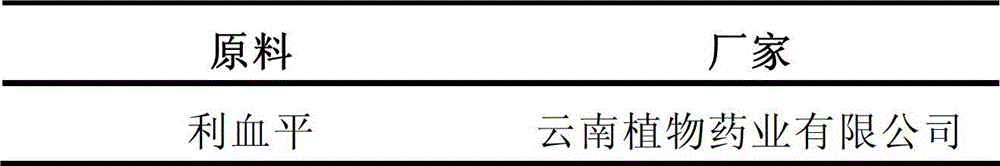
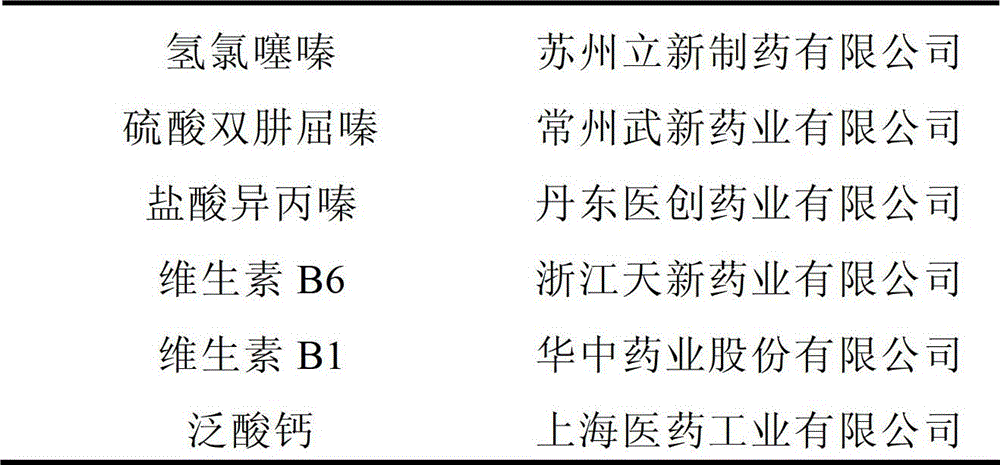
[0077] Prescription: 1000 tablets
[0078]
[0079]
[0080] Preparation Process:
[0081] 1. Weigh an appropriate amount of HPC (hydroxypropyl cellulose) and add water to make a 5% HPC solution for later use;
[0082] 2. Take prescription amount of vitamin B1 and vitamin B6, add 10g starch and 20g pregelatinized starch, granulate with 95% ethanol, dry at 50℃, and granulate with 80 mesh sieve;
[0083] 3. Use the above HPC solution to coat the above particles with a bottom spray, and increase the weight by 40% to obtain particles (1);
[0084] 4. Take the remaining prescription amount of raw and auxiliary materials, granulate with 95% ethanol, make soft materials with a 14-mesh sieve, dry at 50℃, and granulate with 14-mesh sieve to obtain granules (2);
[0085] 5. Mix the particles (1) and (2) into tablets;
[0086] 6. Opadry coating powder is mixed with water to make a 12% coating solution, and the tablet core coating increases by 4%.
[0087] The prepared compound reserpine tablets were ...
Embodiment 2
[0094] Prescription: 1000 tablets
[0095]
[0096]
[0097] Preparation Process:
[0098] 1. Take an appropriate amount of HPC and add water to make a 5% solution, apply a bottom spray powder coating to vitamin B1, take it out after 30% weight gain, and screen out 80-100 mesh particles;
[0099] 2. Take the raw and auxiliary materials of the prescription except vitamin B1, add the above-mentioned vitamin B1 granules, use 95% ethanol to granulate, make soft material with 14 mesh sieve, dry at 50 ℃, granulate with 14 mesh sieve, and compress;
[0100] 3. Opadry coating powder is mixed with water to make a 12% coating solution, and the tablet core coating increases by 4%.
[0101] The prepared compound reserpine tablets were tested according to the quality standards, and the results are as follows:
[0102]
[0103] The test results of influencing factors are shown in the following table:
[0104]
[0105] The results of the accelerated stability test are shown in the following table:
[010...
Embodiment 3
[0108] Prescription: 1000 tablets
[0109]
[0110] Preparation Process:
[0111] Preparation Process:
[0112] 1. Take an appropriate amount of EC and 95% ethanol solution to prepare a 6% EC solution, apply a powder coating to the vitamin B1 under spray, take it out after 30% weight gain, and screen out 80-100 mesh particles;
[0113] 2. Take the raw and auxiliary ingredients of the prescription except vitamin B1, add the above-mentioned vitamin B1 granules, use 95% ethanol to granulate, make soft material with 14 mesh sieve, dry at 50 ℃, granulate with 14 mesh sieve, and compress;
[0114] 3. Opadry coating powder is mixed with water to make a 12% coating solution, and the tablet core coating increases by 4%.
[0115] The prepared compound reserpine tablets were tested according to the quality standards, and the results are as follows:
[0116]
[0117] The test results of influencing factors are shown in the following table:
[0118]
[0119] The results of the accelerated stability tes...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com