Method for removing arsenic in water
A technology for removing water and anions, applied in chemical instruments and methods, water/sewage treatment, water/sludge/sewage treatment, etc. Simple to use effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0025] According to a typical embodiment of the present invention, a method for removing arsenic in water is provided, using the following molecular structure
[0026] Strong base anion exchange resin for removing arsenic in water, where n≥2.
[0027] The method has a high arsenic removal rate, high selectivity in the presence of some coexisting ions, simple process operation, and easy industrialization; and the strong base anion exchange resin has a wide pH range, and the pH is 1-14. Both have high adsorption capacity; fast adsorption rate, short equilibration time, large adsorption capacity; easy to regenerate, and can be recycled and reused.
[0028] Preferably, the strong base anion exchange resin is prepared by the following steps: chloromethylated styrene-divinylbenzene copolymer is prepared by reacting with N-methylimidazole under N, N-dimethylformamide swelling Strong base anion exchange resin with chloride ion as anion.
[0029] According to a typical fact mode of...
Embodiment 1
[0035] Take 5g of chloromethylated styrene-divinylbenzene copolymer resin, add 30mL of DMF to swell for 2h, then add 1.65g of N-methylimidazole, react at 80°C for no less than 36h, filter the resin and wash with ethanol for 3 ~5 times, then put the resin in 30 mL of ethanol and stir at 65°C for 24 hours, filter the resin and dry it under vacuum to obtain a chlorine-type strong base anion exchange resin containing N-methylimidazole structure.
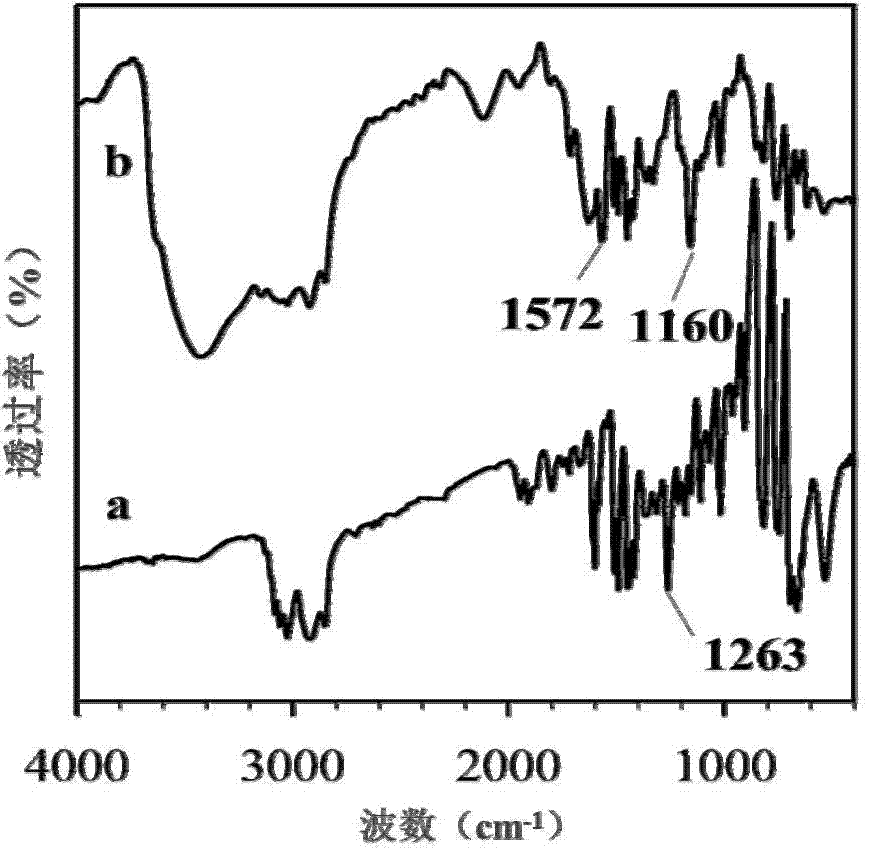
[0036] Such as figure 1 Infrared spectra of the chloromethylated styrene-divinylbenzene copolymer resin (a) and the chlorine form of the strong base anion exchange resin (b) are shown. We can see that the absorption peaks at 1572cm-1 and 1160cm-1 are the skeleton vibration and C-H in-plane bending vibration peaks of the imidazole ring, respectively. This indicates that the Cl in the chloromethylated styrene-divinylbenzene copolymer resin is replaced by amphoteric methylimidazolium chloride groups.
Embodiment 2
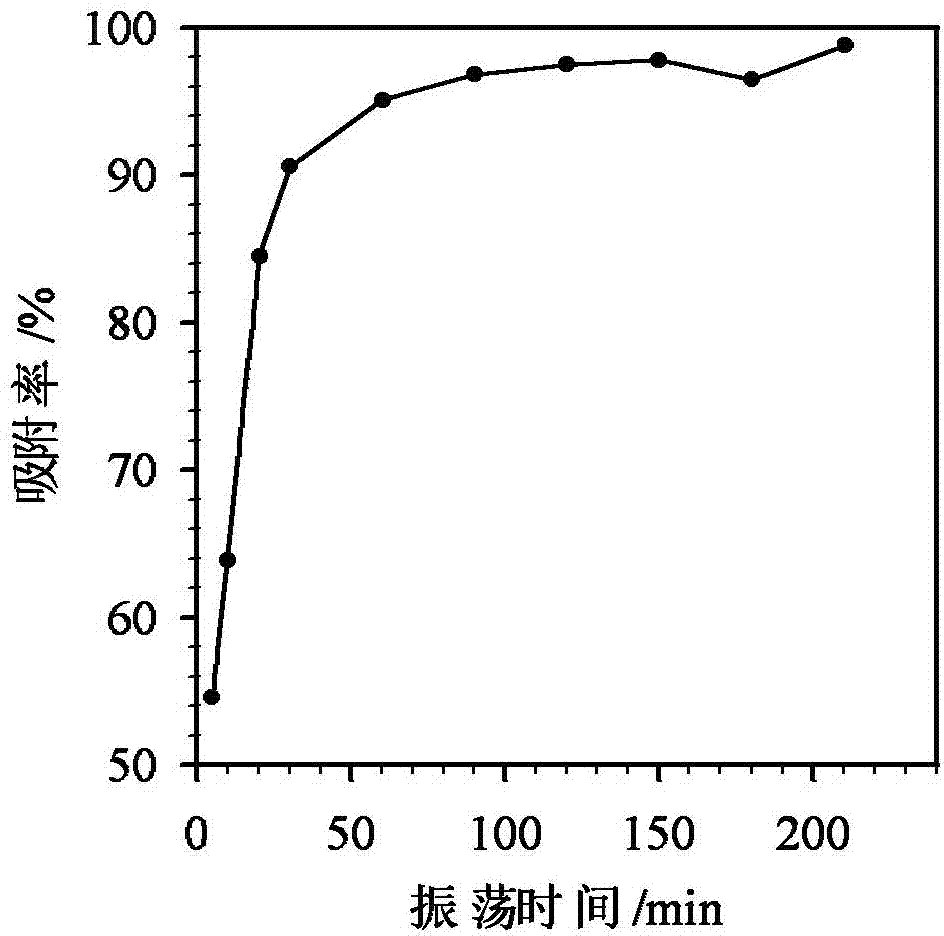
[0038] Take 0.01g of strong base anion exchange resin and put it into a 25mL conical flask with a stopper, add 10mL of arsenate solution containing 10mg / L, and shake for 60min to reach equilibrium. drinking water standards.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com