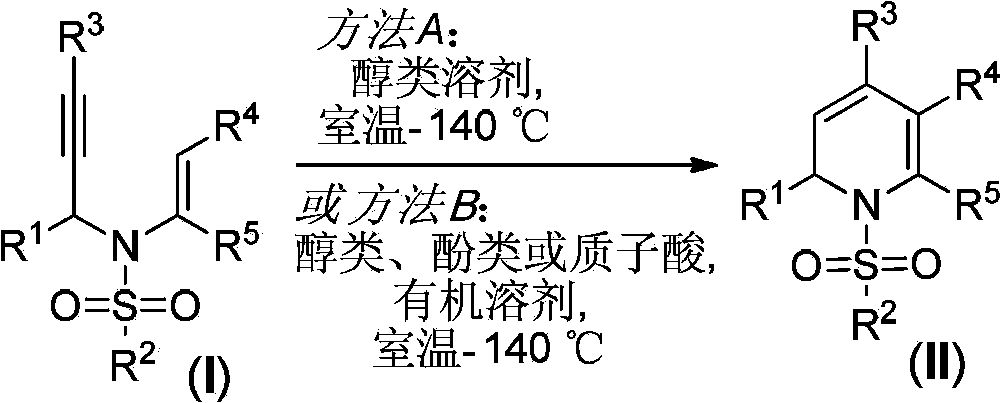
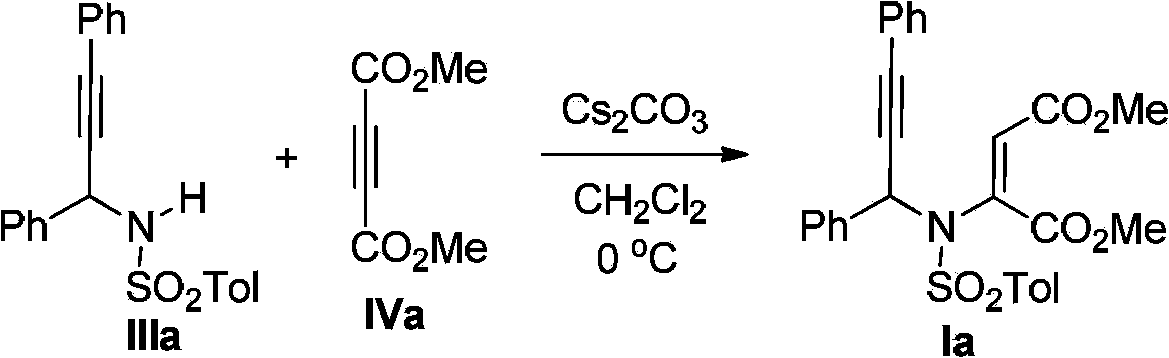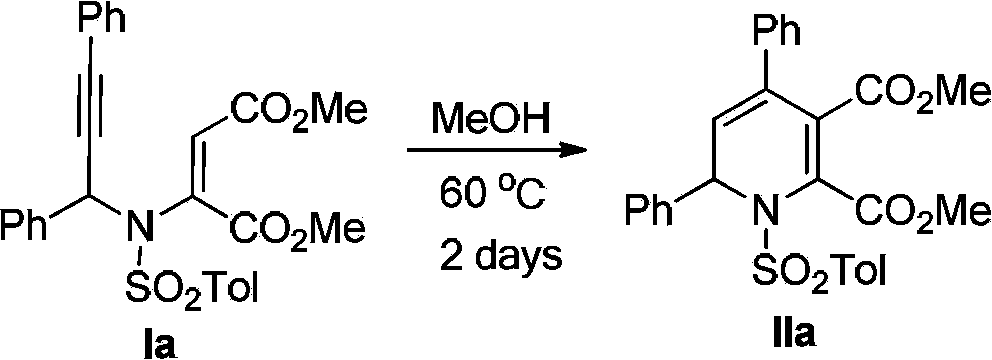Preparation method for 1, 2-dihydropyridine derivative
A technology of dihydropyridine and derivatives, applied in organic chemistry and other directions, can solve problems such as harsh reaction conditions, and achieve the effect of simple reaction operation and high atom economy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Step one (preparation raw material Ia):
[0026]
[0027] The reaction was carried out in the reactor, and after the reactor was evacuated and replaced with argon three times, 5 mmol of N-sulfonyl-allylamine IIIa was added, and 40 mL of freshly distilled CH 2 Cl 2 , then add 5.5mmol of alkyne IVa, and finally add 10mol% of Cs 2 CO 3 , stirred at 0°C for 12 hours. After the reaction, part of the solvent was evaporated until the volume of the solution was one-fifth of the volume of the solution before evaporation, and the sample was loaded for silica gel column chromatography. The eluent was a mixed solvent of petroleum ether:ethyl acetate=10:1, 3-Aza-1,5-enyne Ia is obtained.
[0028] The characterization data for Ia are as follows:
[0029] 1 H NMR (400MHz, CDCl 3 )δ7.92(d,J=8.3Hz,2H),7.64(d,J=7.7Hz,2H),7.40–7.27(m,8H),7.16(dd,J=8.1,1.4Hz,2H), 6.54(s,1H),6.10(s,1H),3.76(s,3H),3.60(s,3H),2.31(s,3H); 13 C NMR (100MHz, CDCl 3 )δ164.9, 164.2, 144.9, 139.7, 135....
Embodiment 2
[0040]
[0041] The reaction was carried out in the reactor, and the reactor was evacuated and replaced with argon, and 0.2mmol (100.7mg) of 3-aza-1,5-enyne Ia was added, 1mL of THF was added, and then 0.02mmol of Hydroquinone (terephthalene Phenol, 10mol%), reacted at 60°C for 2 days. After removing the solvent with a rotary evaporator, the solid was dissolved in dichloromethane and loaded for silica gel column chromatography. The column was washed with an eluent of petroleum ether: ethyl acetate = 10:1 to obtain 95.7 mg of 1,2-bis Hydropyridine IIa, the isolated yield is 95%.
Embodiment 3
[0043]
[0044] Carry out the reaction in the reactor, vacuumize the reactor and replace it with argon, add 0.2mmol (104.3mg) 3-aza-1,5-enyne Ib, then add 2mL methanol, and react at 60°C under the protection of argon 2 days. After removing the solvent with a rotary evaporator, the solid was dissolved in dichloromethane and loaded for silica gel column chromatography. The column was washed with an eluent of petroleum ether: ethyl acetate = 10:1 to obtain 91.9 mg of 1,2-bis Hydropyridine IIb, the isolated yield was 88%.
[0045] The characterization data of Ib are as follows:
[0046] 1 H NMR (400MHz, CDCl 3 )δ7.91(d,J=7.8Hz,2H),7.63(m,2H),7.32(m,5H),7.15(d,J=7.4Hz,2H),7.06(t,J=8.2Hz, 2H),6.50(s,1H),6.12(s,1H),3.77(s,3H),3.63(s,3H),2.32(s,3H);
[0047] 13 C NMR (100MHz, CDCl 3 )δ164.8,164.2,162.9(d,J=244.7Hz),145.0,139.5,135.2,131.7,130.0,129.9,129.8,129.2,128.5,128.4,121.5,118.1,115.7(d,J=21.9Hz),89.8 ,82.7,54.4,53.1,52.2,21.7;
[0048] HRMS Calculated for C 28 h ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Separation yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com