Taxane medicinal precursor
A technology of taxanes and drugs, applied in the direction of peptides, etc., can solve the problem that drug targeting cannot be effectively improved, and achieve good market prospects, good safety, and mild preparation conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 Preparation of Paclitaxel Prodrug Coupled with Liver Cancer Targeting Peptide SP94 via Ester Bond
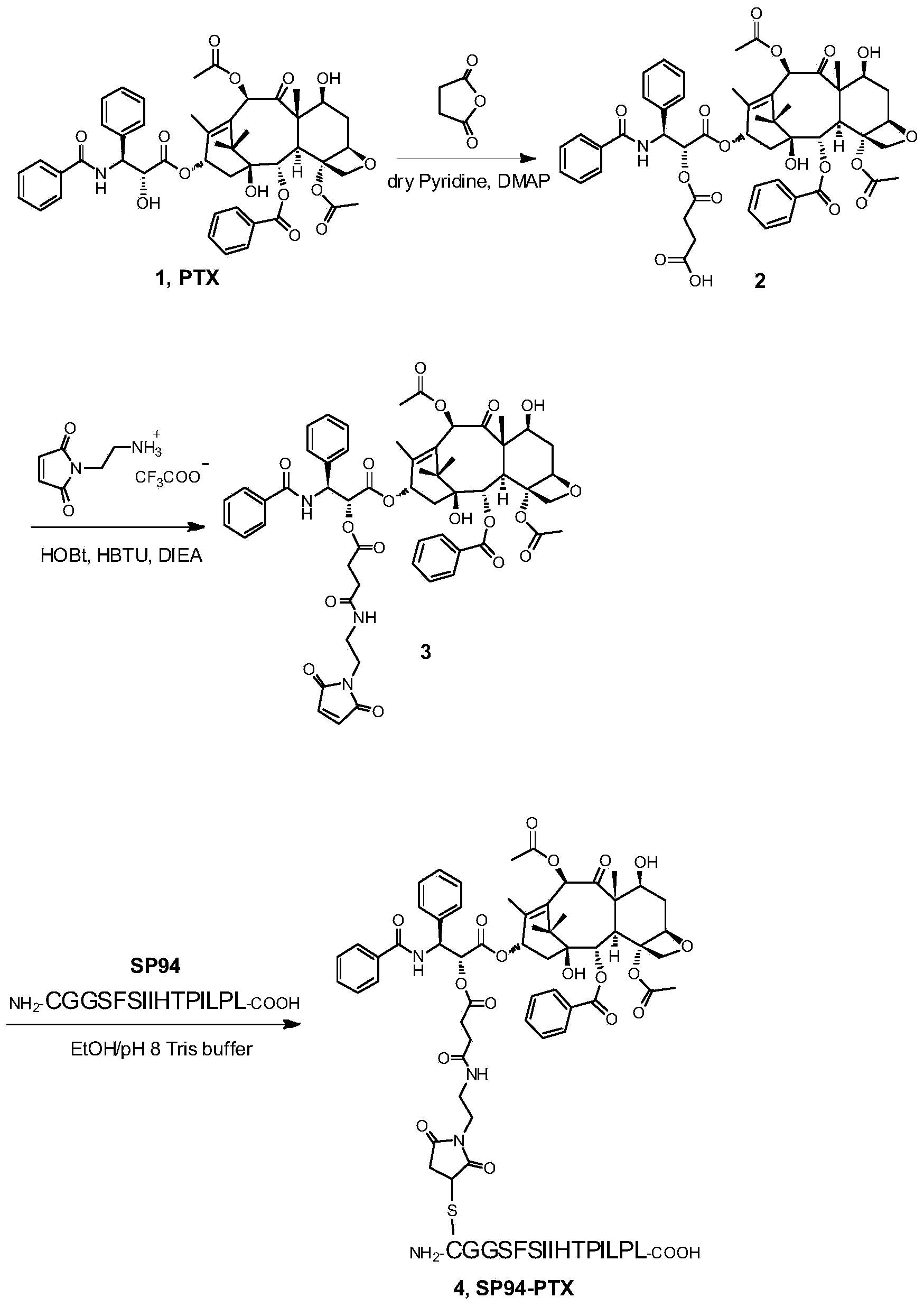
[0042] Taxane prodrugs were prepared by coupling paclitaxel with liver cancer targeting peptide SP94, and the synthetic route was as follows figure 1 shown, including the following steps:
[0043] (1) Synthesis of paclitaxel C’-2 derivative 2
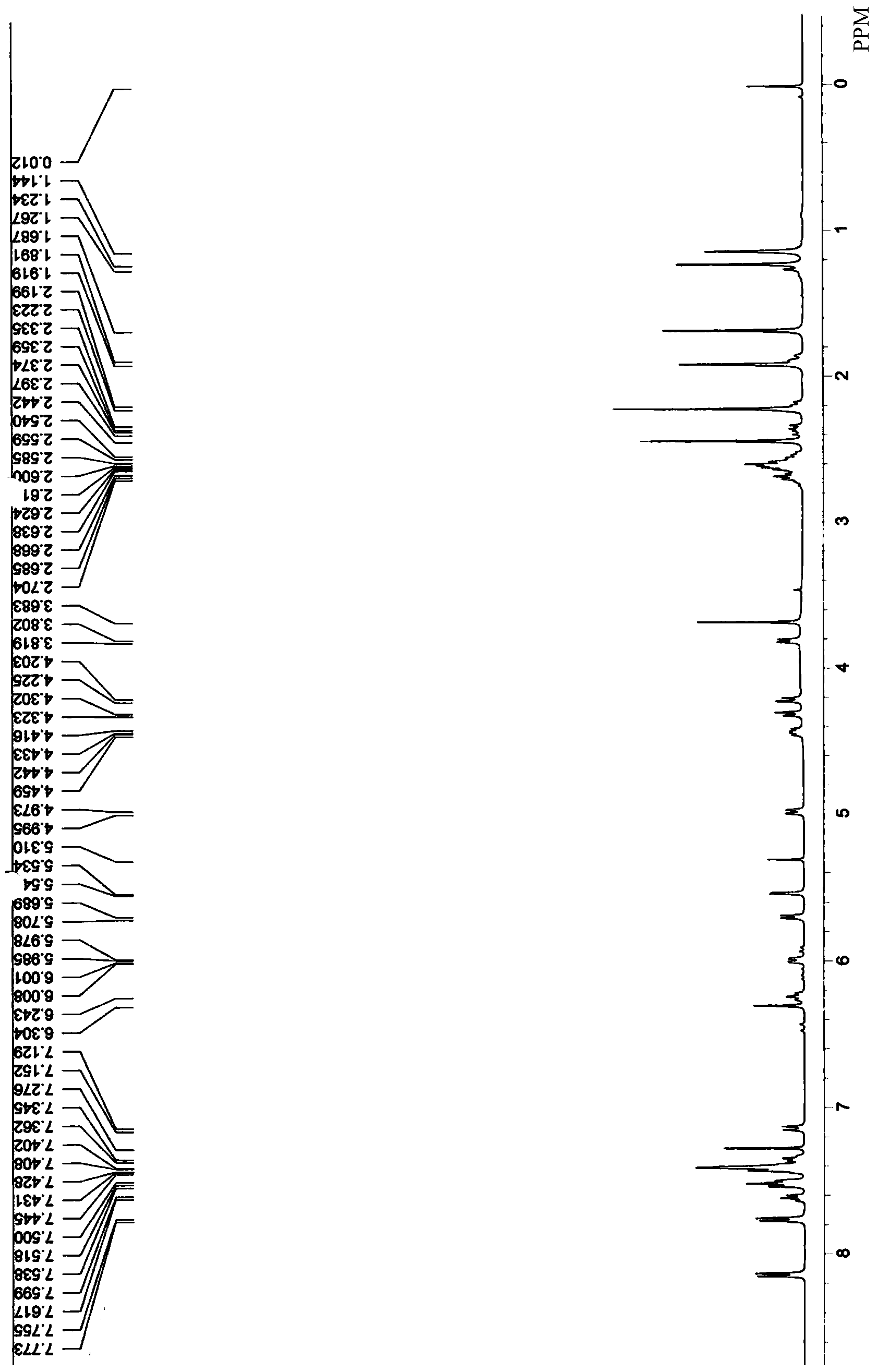
[0044]Add paclitaxel 1 (300mg, 0.35mmol) to the dry reaction bottle, and dissolve it with anhydrous pyridine 2ml; add succinic anhydride (105mg, 1.05mmol) and 4-dimethylaminopyridine DMAP (4.3mg, 0.035 mmol). Stir at room temperature for 2 hours, TLC showed that the reaction was terminated after the end of the reaction, and the pyridine was removed by rotary evaporation under reduced pressure; normal phase silica gel column (dichloromethane: methanol = 20:1) separated to obtain a white solid, and the product yield was 91%. use 1 H-NMR ( figure 2 ) to confirm the target product 2.
[0045] (2) Synthesis of paclitaxe...
Embodiment 2
[0049] Example 2 Preparation of docetaxel conjugated prodrug with liver cancer targeting polypeptide SP94 through carbonate bond and disulfide bond
[0050] Taxane prodrugs were prepared by coupling docetaxel with liver cancer targeting peptide SP94, and the synthetic route was as follows Figure 5 shown, including the following steps:
[0051] (1) Synthesis of compound 9
[0052] Compound 9 was synthesized according to the literature (E.A.Dubikovskaya et al., Overcoming multidrug resistance of small-molecule therapeutics through conjugation with releasable octaarginine transporters, Proceedings of the National Academy of Sciences, 2008, 105(34):12128-12133.).
[0053] (2) Synthesis of docetaxel derivative 11
[0054] Dissolve docetaxel 10 (40 mg, 49.5 μmol) in 2 ml of anhydrous DCM, add compound 9 (22 mg, 59.4 μmol) and DMAP (7.3 mg, 59.4 μmol) to the reaction solution; stir at room temperature for 4 hours, TLC shows that the reaction is complete Then terminate the reactio...
Embodiment 3
[0057] Example 3 Evaluation of Antitumor Drug Efficacy
[0058] In order to evaluate the killing ability of the target product 4 (SP94-PTX) obtained in Example 1 on tumor cells, taking the liver cancer cell line 7721 as an example, the drug efficacy was evaluated by MTT method, and paclitaxel was used as a control. Cytotoxicity results of SP94-PTX4 on liver cancer 7721 see Figure 8 . Depend on Figure 8 It can be seen that after 72 hours of co-culture of water-soluble SP94-PTX and cells, its anti-hepatoma effect in vitro shows that SP94-PTX has the same cytotoxicity as PTX.
[0059]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com